
��16�֣���ԭ������������ƣ�Na2S2O3���ڹ�ҵ������ҽҩ����ҵ�б��㷺Ӧ�ã���ҵ�ձ�ʹ��Na2SO3����ǣ�S������õ���װ����ͼ1��
��֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1������1����K1���ر�K2����Բ����ƿ�м��������ײ����ȣ����Լ���Ϊ��
��
��2������2��ʼ�ձ���C����Һ�ʼ��ԣ���Ӧһ��ʱ�����۵������٣���K2���ر�K1��ֹͣ���ȡ�
��C����Һ�뱣�ֳʼ��Ե�ԭ���������ԣ��� ���� ������������ ��
���������ӷ���ʽ��ʾ��
��װ��B��D�������� ��
����3����C�����û��������ᴿ��ò�Ʒ��
��3�����÷�Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2Ҳ���Ʊ�Na2S2O3������������ͼ2���������������Ӹ��������ӿ�˳��Ϊ�� ��g��h�� �� �� �� ��d��
��4��װ����ʢװ���Լ��ǣ�_____________________________��
��5��Na2S2O3��ԭ�Խ�ǿ����ҵ�ϳ�������ȥ��Һ�в�����Cl2���÷�Ӧ�����ӷ���
ʽΪ�� ����
��6������Ƽ�ʵ�鷽����֤������������Cl2����ԭ����Cl����____________
��
��16 �֣�ÿ��2�֣�
��1��Ũ����
��2�� ��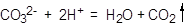 ��
��
�� ����SO2����ֹ��Ⱦ
��3�� a ��g��h�� b �� c �� e �� f ��d��
��4�� Na2CO3��Na2S�Ļ����Һ
��5��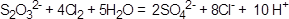
��6�� ȡ������Ӧ�����Һ���Թ��У�����Ba(NO3)2��Һ�����ڲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl����
���������������1��Na2SO3����ǣ�S��������Ƶ���������ƣ�װ��C���Լ�ΪNa2CO3��Һ����ۣ������Լ�����ͭƬ��Ӧ����SO2�����Լ���ΪŨ���ᡣ
��2����Na2S2O3��������Һ�в����ȶ����ڣ�����C����Һ�������ԣ�������Ӧ��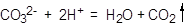 ��
��
��β���е�SO2�Ǵ�����Ⱦ�����װ��B��D�������ǣ�����SO2����ֹ��Ⱦ��
��3��a��g��h����Ӧ����ʱͨ��a����β�����գ�Ȼ���b��c������һ��������װ�ã���e�������У�SO2��װ�����Լ�������Ӧ��ȡ��������ƣ�Ȼ��f��d������β���������ʽӿ�˳��Ϊ��a��g��h��b��c����e��f��d��
��4����Ϊ��ȡ��������Ƶ�װ�ã�ͨ������ͨ��SO2����װ����ʢװ���Լ���������Ӧ�Na2CO3��Na2S�Ļ����Һ��
��5��Na2S2O3��ԭ�Խ�ǿ����Cl2����������ԭ��Ӧ������SO42?��Cl?��H+�����ݻ��ϼ���������ƽ�ɵ����ӷ���ʽ��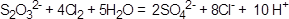 ��
��
��6����Ӧ�����Һ����SO42?�������Cl?�ļ��飬����Ӧ����������Ba(NO3)2��Һ��ȥSO42?��Ȼ���ٽ���Cl?�ļ��飬��ʵ�鷽��Ϊ��ȡ������Ӧ�����Һ���Թ��У�����Ba(NO3)2��Һ�����ڲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl����
���㣺���⿼��ʵ�鷽���ķ�������ơ����ӷ���ʽ����д�����ӵļ��顣


 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ļ��������ڴ����ŵ���ѭ�������������������������������������������������������ͼ����Ȼ���е���ѭ��ͼ����ش��й����⡣
��1���ӿ����л�ȡ����������������Ŀ�ꡣ�����й�˵����ȷ����_________������ţ���
a��ͼ�Т٢ڢۢܢݶ�������Ȼ��Ĺ̵�����
b���ڹ��̢��У���Ԫ�صĻ��ϼ۽���
c����Ӧ�ڵĻ�ѧ����ʽ��N2 +O2  2NO
2NO
d���¹���ѧ�ҹ��������˺ϳɰ����գ�����Ҫ��Ӧ����2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
��2������������ɵõ����ᣬ���������백�γɺ������ܸߵķ���NH4NO3��ʩ����������ʵ�ַ���Ȼ��ʽ�ĵ�ѭ���������ַ��ϲ�������������ʹ��ã�����ή�ͷ�Ч����ԭ����_________________________________�������ӷ���ʽ��ʾ����
��3�������Ե�ѭ�����������Ե�Ӱ�졣����β���е�NO������ɹ⻯ѧ����������֮һ����NO�����������Ŷ��ص��������ã�����Ϊ�����Ƿ��ӡ�������λ��ѧ��������о��ɹ������1998��ŵ��������������ʵ˵������Ӧ��֤�ؿ�����ѧ���ʵ����á��Ƽ���Ա�Ѿ��ҵ���һЩ���NO�ŷŵķ�����������β���ŷŹ��а�װһ����ת�������ɽ�β������һ���к�����CO��NO��Ӧת��Ϊ�����е����ֳɷ֡������ڴ�������������������õĻ�������ͼ��ʾ��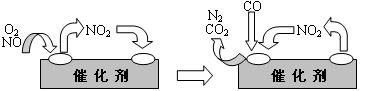
д�������仯�е��ܻ�ѧ��Ӧ����ʽ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�Ӵ������������Ȱ�SO2��������SO3��Ȼ����Ũ�������յõ���SO3��ȡ��Ʒ��ij������������ʱ������Ӵ��ҵ�ԭ�����ɷ�ΪSO27%��O2 11%��N2 82%�������������
��1�������״����10 m3ԭ�����е�SO2���ʵ���______________mol��
��2�������״����1 0m3ԭ���������� ǧ�ˡ�
��3����SO2��ת����Ϊ99��2%������Ӵ��ҵ�����������SO3��������� ��
��4�����Ӵ��ҵ����������к�6��72���������������SO3���ѳ��������ͽ�����������
98��3%���������գ��ɵõ�������H2SO4����H2SO4��SO3�Ļ������к���������Ϊ20����SO3������������1000 m3�������壨������Ϊ��״��������Ҫ��98��3%����������� ǧ�ˡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ���ҿ��������·�����ȡ���������壺��
2NaBr+MnO2+2H2SO4 Na2SO4+MnSO4+Br2��+2H2O
Na2SO4+MnSO4+Br2��+2H2O
������ͼʵ��װ��,��պͻش����⣺��������������������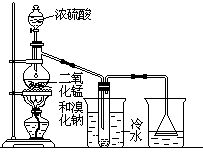
��1��װ��(��)���ձ�����ˮ�����������______ ,װ��(��)���ձ���Һ��������� ______________��
��2�����д�ʵ��ʱ,��ƿ�ڵ���Ƥ�������������ס,����Ƥ�����ӵ��������ܿ�Ҫ�����,������Ϊ ____________________________��
��3��װ��(��)�ձ���ʹ�õ���©���ɷ�ֹҺ�嵹��,�Լ�����ԭ��
________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣�һ������Ũ����������Zn��ַ�Ӧʱ��SO2��H2���ɡ�ijУ��ѧ�о���ѧϰС��Ӷ��Է���Դ������о���
��ͼ��װ��ʵ��װ�ã����Թ�A���۲쵽C��D��E�о������ݲ�����������������٣�Ʒ����Һ��ɫ��D���ȳ��ֻ��ǣ��������ʧ����Ӧ�ϳ�ʱ���C��D��E�е��������ֻ��������ӡ�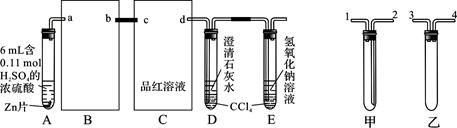
�Իش��������⣺
��1���Ӽס�����ѡ����ʵ�װ������B��C�У���������ȷ���ӣ�a��______��______��b��c��______��______��d��D��E��֧�Թ���CCl4��������________________________��
��2����֤��Ũ�������ǿ�����Ե�ʵ������Ϊ_______________________________��ʵ������У�Ũ�������ǿ�����Եķ�Ӧ����ʽ�ǣ�
____________________________________________________________________
��3����Ӧ�ϳ�ʱ����������ֻ��������ӵ�ԭ����________________________________
____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������16�֣���ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�顣
��ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺
�������Ӻ�װ�ã����������ԣ������Լ���
�ڼ���A�Թ�ֱ��B��Ʒ����ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿�����뿪Һ�档
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϩ��ƾ��ƺ���Ϊ�е���D�Ĵ��ڣ�B�е�Һ�岻�ᵹ������ԭ���� ��
��3�����װ��ǰ������������Ϳ�ʹװ���в���������ȫ�����գ�Ӧ����ȡ�IJ����� ��
���͵�NaHSO3��Һ������ȩ�����ӳɷ�Ӧ������ˮ���ԵĦ�-�ǻ������ơ������ķ�ӦΪ��
R-CHO + NaHSO3 R-CH��OH��SO3Na
R-CH��OH��SO3Na
��Ӧ�ǿ���ģ���ͨ����������70%--90%������Ӧ����ת����
��4�����â���װ����ȡ����NaHSO3��Һ��Ӧ��ȡ��ʩ����װ�ý��в��ָı䡣���ִ�ʩ�ǣ�_____________________________________________________________________��ȷ����NaHSO3���ɵ�������:_______________________________________.
��5�����屽�л���������ȩ,���������ʳ�ȥ,�ɲ��õ��Լ���:__________,�����ķ�����:_____________________.
��6������CH3-CH(OH)SO3Na ˮ��Һ�м�����������,�л���ת��Ϊ:___________,����ת�������������ķ�������__________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(14��)������ͼװ�ý���ľ̿�ۺ�Ũ����ķ�Ӧ�������ļ��顣
��֪���Ϻ�ɫ�����Ը��������Һ�������������������ԭ��Ӧ�������Ը�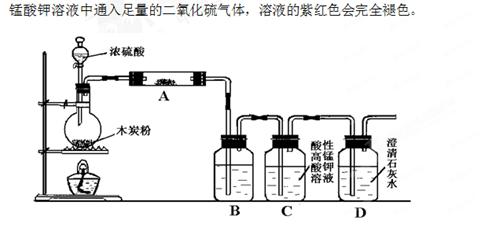
��1����װ������װ�ú�����Ҫ���еIJ����ǣ� ��
��2��д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ�� ��
��3����ͼ�е�װ�ü���������Ӧ��ȫ�����д������������ʾ��������Ӧ�����Լ������Ƽ������ã�A�м�����Լ��������� �� ��B�м�����Լ��������� �� ��
��4��ʵ��ʱ��C��Ӧ�۲쵽ʲô���ſ���˵�������˶�����̼�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��һ����ȡ������������Ϊԭ�Ͻ����ض���Ӧ��װ�á�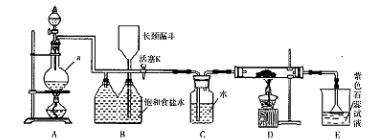
��l��A����������װ�ã�д�����еĻ�ѧ��Ӧ����ʽ ��
��2��a����������Ϊ ��
��3��ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ�����K����������������װ�ã��ٵ�ȼD���ƾ��ƣ�������Eװ�á�Dװ�õ�Ӳ�ʲ�������ʢ��̿�ۣ���Ӧ����ΪCO2��HC1��д��D�з�Ӧ�Ļ�ѧ����ʽ ��װ��C�������� ��
��4����E������©����������л���ɫ������ɫʯ����Һ����ɫ�仯Ϊ ��
��5������E���ձ�����Һ��Ϊ����ʯ��ˮ����Ӧ����������Ϊ ������ţ���
���а�ɫ�������ɣ����ް�ɫ�������ɣ��������ɰ�ɫ�����������ɫ������ʧ��
��6��D����Ӧ��Ϻرջ���K����ȥ�ƾ��ƣ��������ȵ����ã�A������Cl2
��������װ��B�е������� ��������װ��B�и��������ԣ����ܵ�
ԭ���� ������ԭ��ʱ���÷���ˮ��ʪ��ĵ⻯�ص�����ֽ�ȣ�����պ
��Ũ��ˮ�IJ��������ʱ�۲쵽�а��̲�����д����������ԭ��Ӧ�Ļ�ѧ����
ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
��̼���� ��̼������ ��̼����� ���Ȼ�� ����ʯ�� ����������
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�� ����������ţ�
��2��Bװ�õ�����Ϊ
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е� �������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com