
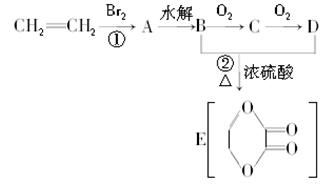
 »„¶ÆÓ¢ÓļĻµĮŠ“š°ø
»„¶ÆÓ¢ÓļĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

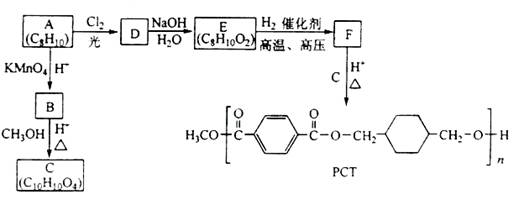
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
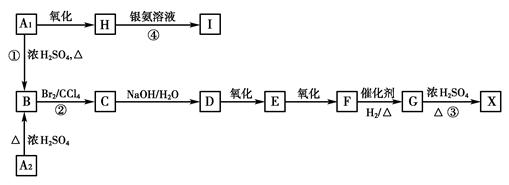
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
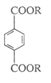
| A£®MÄÜŌŚĻ”ĮņĖįČÜŅŗ·“Ӧɜ³É“¼ŗĶōČĖį | |
| B£®Imol MŌŚ“߻ƼĮ”¢¼ÓČČĢõ¼žĻĀ×ī¶ąÓė5mol H2·“Ó¦ | |
| C£®1mol MÄÜÓė2mol NaOH·¢ÉśĖ®½ā·“Ó¦ | D£®MÓė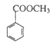 »„ĪŖĶ¬ĻµĪļ »„ĪŖĶ¬ĻµĪļ |
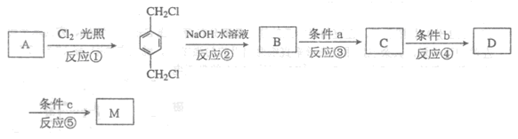
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
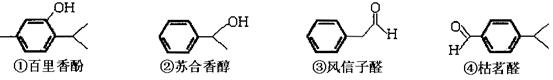 Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ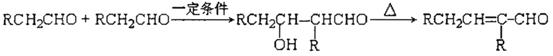
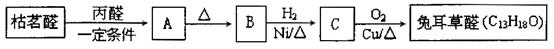
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¾ŪĀČŅŅĻ©µÄµ„ĢåŹĒCH3CH2Cl |
| B£®¾ŪĀČŅŅĻ©ŹĒøß·Ö×Ó»ÆŗĻĪļ |
| C£®¾ŪĀČŅŅĻ©Äܹ»Ź¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ« |
| D£®¾ŪĀČŅŅĻ©±£ĻŹÄ¤×īŹŹŗĻÓĆĄ“°ü×°Źß²Ė”¢Ė®¹ū¼°ŹģŹ³ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| A£®Éś²śŅ»“ĪŠŌ·¹ŗŠµÄµķ·Ū | B£®ŃņĆ« | C£®Å£ÓĶÖ¬ | D£®PVCĖÜĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
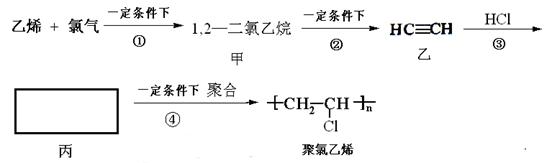
 ӣ
”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
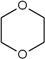 £©”£
£©”£ ÉŌÓŠĻćĪ¶£¬Ö÷ŅŖÓĆ×÷ČܼĮ”¢Čé»Æ¼Į”¢Č„¹ø¼ĮµČ£¬Ęä·Ö×ÓŹ½ĪŖ ”£
ÉŌÓŠĻćĪ¶£¬Ö÷ŅŖÓĆ×÷ČܼĮ”¢Čé»Æ¼Į”¢Č„¹ø¼ĮµČ£¬Ęä·Ö×ÓŹ½ĪŖ ”£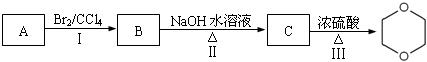
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com