
| ӦȡŨ��������/ml | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
| | | |
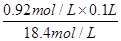 ��0.005L��5.0ml������ӦȡŨ����������5.0ml������100ml���ᣬ����Ҫ100ml����ƿ�������Ҫ��Ͳ��ȡŨ���ᣬϡ��Ũ�������ձ���ϡ�ͺ�ת�ƻ���Ҫ������������ʱ����Ҫ��ͷ�ιܡ�
��0.005L��5.0ml������ӦȡŨ����������5.0ml������100ml���ᣬ����Ҫ100ml����ƿ�������Ҫ��Ͳ��ȡŨ���ᣬϡ��Ũ�������ձ���ϡ�ͺ�ת�ƻ���Ҫ������������ʱ����Ҫ��ͷ�ιܡ�

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ħ�������ʵ������ĵ�λ | B��̼��Ħ������Ϊ12g |
| C�������ӵ�����ԼΪ6.02��1023 /mol | D������Ħ�����Ϊ22.4L/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����1��=��2��=��3�� | B����1��=��2��>(3) |
| C����1��>(2)��(3) | D����2��>(1)=(3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������ᾧ | B������ | C�����������Na2CO3��Һ | D����ˮ�ܽ� E������������ռ���Һ F���������ϡ���� G�����������Ba(NO3)2��Һ H�����������ϡ���� I�����������BaCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.5 mol | B��0.6 mol | C��0.7 mol | D��0.8 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȡ100mL5mol/LH2SO4������400mLˮ |
| B��ȡ200mL5mol/LHCl����ˮϡ����500mL |
| C��ȡ200 mL5mol/LH2SO4����ˮϡ����500mL |
| D��ȡ100 mL5mol/LHNO3����ˮϡ����500mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com