
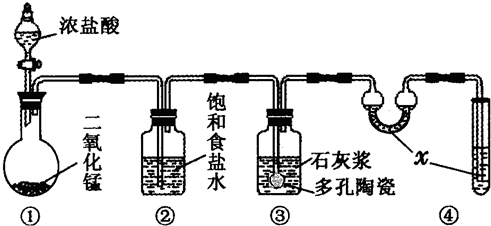
��15�֣�ij�о���ѧϰС��������ͼ��ʾװ���о��Ҵ����������ķ�Ӧ����ش��������⣺
��1��װ�����Թ�B�������� ��
��2��ʵ���пɹ۲쵽ʯӢ��A�е�����Ϊ ��
��3����Ӧֹͣ��ȡ���Թ�C�ھƾ����ϼ��������ڣ��ɹ۲쵽�к�ɫ����������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��Ϊ�˲ⶨ��Ӧ��ʯӢ��A����������Ԫ�صĺ�������������ʵ�飺
��i�� ��������õ��IJ����������ձ�������������ͷ�ιܡ� ��
��ii�������йز���ܵIJ�����˵����ȷ���� ��
a���ζ������п����õ�����Һ��Ϊָʾ��
b���ζ���������ˮϴ�Ӻ����ֱ��װҺ
c����ƿ����Ҫ�ô���ҹ��ϴ
d���ζ������У��۾�ע�ӵζ�����Һ��仯
e���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
��iii���ɿ�ͼ�����ݼ��㣬�ɵ�ʯӢ��A����������Ԫ�صİٷֺ���Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
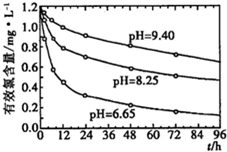
| ����� | 1 | 2 | 3 |
| KI��Һ���/mL | 19.98 | 20.02 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
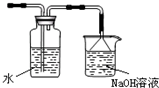
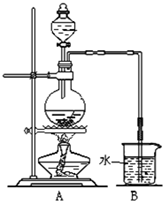 ��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
| ||
| ||
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 1 |
| 2 |
| 1 |
| 2 |
| ||
. |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com