

���� ʯ���ѻ����ڻ�ѧ��Ӧ����ѭԭ�Ӹ����غ㣬C16H34$��_{��}^{����}$C8H18+�ף����Լ���ʽΪ��C8H16�����ݷ���ʽ��C8H16$��_{��}^{����}$4�ң����ԭ�Ӹ����غ��֪����Ϊ��C2H4������ʵ��װ��ͼ��֪��A�в�������ϩ��B�е���ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ����ϩ�����������Һ�������ɶ�����̼��C����ҺҲ��ɫ�����ɵĶ�������̼ʹD�еij���ʯ��ˮ��룬װ�����������ϩ��������ˮ���ռ���
��1������ԭ�Ӹ����غ��жϼס������ʣ�
��2����ϩ����̼̼˫�����ܹ����巢���ӳɷ�Ӧ��ˮ��ɫ��
��3����ϩ���л�ԭ�ԣ��ܹ������Ը������������
��4��������̼���������Ʒ�Ӧ����̼��Ƴ�������ϩ����������ȡ����Ӧ�����Ȼ�����廯�⣬�廯������ˮ�����ԣ�
��5����д������C4H10��ͬ���칹�壬Ȼ�������ԭ�ӵ��������һ�ȴ����������ȷ����
��6���л��ﺬ̼��Խ�ߣ�ȼ�ջ���Խ��������ԽŨ��
��� �⣺��1��ʯ���ѻ����ڻ�ѧ��Ӧ����ѭԭ�Ӹ����غ㣬C16H34$��_{��}^{����}$C8H18+�ף����Լ���ʽΪ��C8H16�����ݷ���ʽ��C8H16$��_{��}^{����}$4�ң����ԭ�Ӹ����غ��֪����Ϊ��C2H4���ṹ��ʽΪ��CH2=CH2��
�ʴ�Ϊ��C8H16��CH2=CH2��
��2����ϩ����̼̼˫�����ܹ����巢���ӳɷ�Ӧ��ʹ��ˮ��ɫ������ʽΪ��CH2=CH2+Br2��CH3Br-CH3Br��
�ʴ�Ϊ����ˮ��ɫ��
��3����ϩ���л�ԭ�ԣ��ܹ������Ը��������������������Ӧ��5CH2=CH2+12KMnO4+18H2SO4��10CO2+12MnSO4+28H2O+6K2SO4�����¸��������Һ��ɫ��
�ʴ�Ϊ����ɫ��ɫ�����dz���� ������Ӧ��
��4����ϩ�����Ը��������Һ��Ӧ����������̼��������̼�ܹ����������Ʒ�Ӧ����Ӧ�����ӷ���ʽΪCa2++2OH-+CO2=CaCO3��+H2O������̼��Ƴ�����D��װ�г����ʯ��ˮ���ɸ���D�е�ʵ��������жϸ������Ƿ���ʵ��
�ʴ�Ϊ��D��
��5������ʽΪC4H9Cl��ͬ���칹���У�������4��̼ԭ�ӵģ�CH3CH2CH2CH2Cl��CH3CH2CHClCH3��������3��̼ԭ�ӵģ�CH3CH��CH3��CH2Cl��CH3CCl��CH3��2��
����4�������
�ʴ�Ϊ��4��
��6����ϩ��̼�����ڼ��飬����ȼ�ջ������������к��̣�
�ʴ�Ϊ����ϩ�к�̼���ߣ�����ȼ�ղ���ȫ��
���� ���⿼������ϩ��ʵ�����Ʒ��Լ�����ļ��飬��Ϥ�ѻ���ԭ����ԭ�Ӹ����غ���ɡ���ϩ�������ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
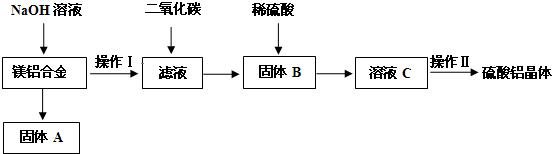
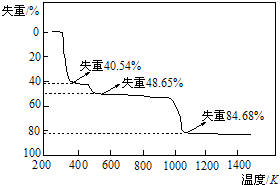
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
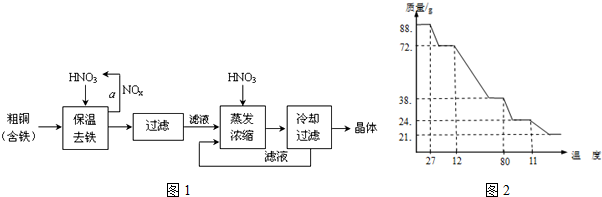
| �������↑ʼ����ʱ��pH | �������������ȫʱ��pH | |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
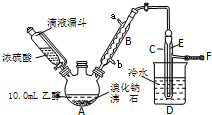 ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ������飬�䷴Ӧԭ����ʵ���װ�����£���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã���
ʵ�������Ҵ���Ũ������廯�Ʒ�Ӧ���Ʊ������飬�䷴Ӧԭ����ʵ���װ�����£���Ӧ��Ҫ���ȣ�ͼ��ʡȥ�˼���װ�ã���| �Ҵ� | ������ | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ���ɫҺ�� |
| �ܶ�/��g•cm-3�� | 0.79 | 1.44 | 3.1 |
| �е�/�� | 78.5 | 38.4 | 59 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Լ���Ϊָʾ��������Һ�ɺ�ɫ���ɫ | |
| B�� | �ζ�ǰ��ʽ�ζ��ܼ��촦�����ݣ��ζ���������ʧ | |
| C�� | �ζ��ܶ���ʱ���ζ�ǰ���ӣ��յ�ʱ���� | |
| D�� | ��ʱ��ƿ�е�Һ�ν����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ȶ��ԣ�HBr��HCl | B�� | ���ԣ�Al��OH��3��Mg��OH��2 | ||
| C�� | �����ԣ�O��F | D�� | ��ԭ�ԣ�S2-��O2- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com