
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
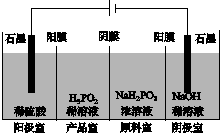
��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
(1)H3PO2 H2PO
H2PO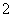 ��H����(2)�٣�1����H3PO4�������Ρ�������
��H����(2)�٣�1����H3PO4�������Ρ�������
(3)2P4��3Ba(OH)2��6H2O��3Ba(H2PO2)2��2PH3��
(4)��2H2O��4e��===O2����4H��
�������ҵ�H��������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO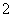 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO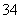 ��H2PO
��H2PO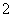 ��H3PO2������
��H3PO2������
[����] (1)H3PO2ΪһԪ���ᣬ����뷽��ʽΪH3PO2 H����H2PO
H����H2PO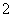 ��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO
��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO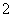 ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO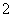 ��H3PO2ʧ���ӷ���������Ӧ�������������л���PO
��H3PO2ʧ���ӷ���������Ӧ�������������л���PO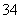 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й���Һ��ɵ�������������(����)
A����ɫ��Һ�п��ܴ�������Al3����NH ��Cl����S2��
��Cl����S2��
B��������Һ�п��ܴ�������Na����ClO����SO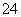 ��I��
��I��
C����������Һ�п��ܴ�������Na����K����Cl����HCO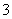
D��������Һ�п��ܴ�������Fe3����K����Cl����SO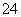
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������������Ӧ�ɻ�û��������C4H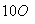
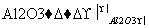
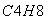
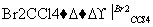
 �����Ľṹ��ʽ��������(����)
�����Ľṹ��ʽ��������(����)
A��CH3CH(CH2Br)2���������� B��(CH3)2CBrCH2Br
C��C2H5CHBrCH2Br D��CH3(CHBr)2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�������Һ������Ũ�ȹ�ϵʽ��ȷ����(����)
A��pH��5��H2S��Һ�У�c(H��)��c(HS��)��1��10��5 mol��L��1
B��pH��a�İ�ˮ��Һ��ϡ��10������pH��b����a��b��1
C��pH��2��H2C2O4��Һ��pH��12��NaOH��Һ���������ϣ�c(Na��)��c(H��)��c(OH��)��c(HC2O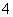 )
)
D��pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c(Na��)���٣��ڣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij�¶���CH3COOH��NH3��H2O�ĵ��볣����ȣ�����10 mLŨ��Ϊ0.1 mol��L��1��CH3COOH��Һ�еμ���ͬŨ�ȵİ�ˮ���ڵμӹ�����(����)
A��ˮ�ĵ���̶�ʼ������
B.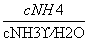 �������ټ�С
�������ټ�С
C��c(CH3COOH)��c(CH3COO��)֮��ʼ�ձ��ֲ���
D�������백ˮ�����Ϊ10 mLʱ��c(NH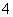 )��c(CH3COO��)
)��c(CH3COO��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��Ũ��ˮ�еμ�FeCl3������Һ���Ƶ�Fe(OH)3����
B��CH3COONa��Һ�еμ�����Ũ�����c(CH3COO��)����
C��Ca(HCO3)2��Һ�����NaOH��Һ��Ӧ�ɵõ�Ca(OH)2
D��25 ��ʱCu(OH)2��ˮ�е��ܽ�ȴ�������Cu(NO3)2��Һ�е��ܽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ѧ�кܶࡰ���ɡ����������÷�Χ�����и����йء����ɡ��Ƴ��Ľ�����ȷ����(����)
| ѡ�� | ���� | ���� |
| A | ��ǿ�������ȡ������ | ��������Һ����ȡ���� |
| B | ��Ӧ��Ũ��Խ��Ӧ����Խ�� | �����£���ͬ����Ƭ�зֱ����������Ũ��ϡ���ᣬŨ��������Ƭ���ܽ��� |
| C | �ṹ��������Ƶ����ʣ��е�����Է���������������� | NH3�ķе����PH3 |
| D | �ܽ��С�ij��������ܽ�ȸ�С�ij���ת�� | ZnS�����еμ�CuSO4��Һ���Եõ�CuS���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ��ش��������⣺
(1)ʵ������ͨ����NaOH��Һ����ϴ�����ᴿ������100 mL 3 mol/L��NaOH��Һ���ձ�״����4.48 L CO2ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ______________________________________________��
(2)�����£���һ�������0.1 mol/L�Ĵ�����Һ�м�ˮϡ�ͺ�����˵����ȷ����________��
A����Һ�е������ӵ���Ŀ����
B������ĵ���̶�����c(H��)������
C����Һ�� ����
����
D����Һ��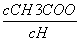 ��С
��С
(3)�ٳ����½�0.15 mol/L��ϡ����V1 mL��0.1 mol/L��NaOH��ҺV2 mL��ϣ�������Һ��pHΪ1����V1��V2��__________(��Һ����ı仯���Բ���)��
�ڳ���������Һ��pH��3��HA��ҺV1 mL��pH��11��NaOH��ҺV2 mL��϶��ã�������˵����ȷ����__________��
A������Ϻ���Һ�����ԣ���c(H��)��c(OH��)��2��10��7mol/L
B����V1��V2����Ϻ���Һ��pHһ������7
C������Ϻ���Һ�����ԣ���V1һ������V2
D������Ϻ���Һ�ʼ��ԣ���V1һ��С��V2
(4)�����£�Ũ�Ⱦ�Ϊ0.1 mol/L������������Һ��pH�����ʾ��
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
�ٸ��ݱ������ݣ���Ũ�Ⱦ�Ϊ0.01 mol/L���������������Һ�ֱ�ϡ��100����pH�仯��С����__________��
A��HCN B��HClO
C��H2CO3 D��CH3COOH
�ڸ����������ݣ��ж����з�Ӧ���Գ�������________��
A��CH3COOH��Na2CO3===NaHCO3��CH3COONa
B��CH3COOH��NaCN===CH3COONa��HCN
C��CO2��H2O��2NaClO===Na2CO3��2HClO
D��NaHCO3��HCN===NaCN��H2O��CO2��
(5)�������ӿ�ʼ����ʱ��pH���±���
| ���� | Fe2�� | Cu2�� | Mg2�� |
| pH | 7.6 | 5.2 | 10.4 |
������ͬŨ��Cu2����Mg2����Fe2������Һ�еμ�NaOH��Һʱ��________(�����ӷ���)�ȳ�����Ksp[Fe(OH)2]________Ksp[Mg(OH)2](�>����������<��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com