
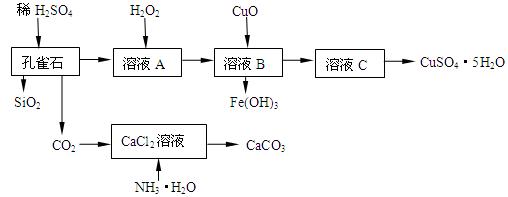
| A��KMnO4��Һ | B��Fe�� | C��Na2CO3��Һ | D��KSCN��Һ |


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �������������յ�֪ʶ�ش�
�������������յ�֪ʶ�ش�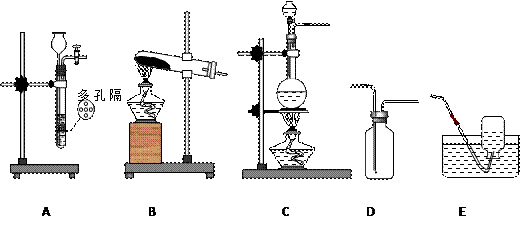
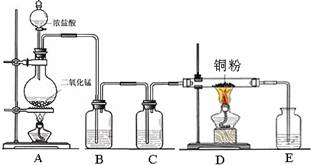
 ��ȡCl2��NH3�Ļ�ѧ����ʽ��
��ȡCl2��NH3�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ ��
��Ӽס�����λͬѧ��Ƶ�װ����ѡ��һ����Ϊʵ������ȡ����������װ�ã�Ӧѡ���װ���� ��ѡ��ס����ҡ�������ͬѧ����˱�װ�ã������θ���ܴ��沣���ܣ����������������⣬��һ��Ҫ������ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 B��������������ˮ
B��������������ˮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
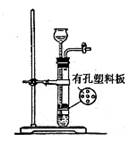
| A��O2���������̺�˫��ˮ | B��SO2���������ƺ�ϡ���� |
| C��CO2������ʯ��ϡ���� | D��H2S����������ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������NaOH�Ѿ����� |
| B��������ƿ��ˮʱҺ����ڿ̶���ҡ�� |
| C��������NaOH��Һ�������ձ��� |
| D��������ƿ��ˮʱ�۾�һֱ����Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com