
µ„ÖŹŌŚµćČ¼Ģõ¼žĻĀ·“Ӧɜ³ÉX”¢Y”¢Z”¢WĖÄÖÖ»ÆŗĻĪļ£¬Ęä×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾”£
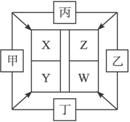
ÓÖÖŖ¢Ł¼×”¢ŅŅ”¢±ū¾łĪŖĒ°ČżÖÜĘŚŌŖĖŲµÄµ„ÖŹ£¬³£ĪĀĻĀ¾łĪŖĘųĢ¬£»¶”ŹĒČÕ³£Éś»īÖŠŅ»ÖÖ³£¼ūµÄ½šŹō”£
¢Ś³£ĪĀĻĀXŹĒĪŽÉ«ŅŗĢ壬YŹĒŗŚÉ«¹ĢĢ唣
¢Ū±ūŌŚŅŅÖŠČ¼ÉÕ·¢³ö²Ō°×É«»šŃę£¬¶”ŌŚŅŅÖŠČ¼ÉÕÉś³É×Ų»ĘÉ«µÄŃĢ£¬WµÄĖ®ČÜŅŗ³Ź»ĘÉ«”£
ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)¶”ŌŚ¼×ŗĶXĶ¬Ź±“ęŌŚµÄĢõ¼žĻĀ£¬æÉ·¢Éśµē»ÆѧøÆŹ“£¬Š“³öÕż¼«µÄµē¼«·“Ó¦Ź½£ŗ____________________________________________”£
(2)½«ÉŁĮæWµÄ±„ŗĶČÜŅŗµĪČėČȵÄXÖŠ£¬·“Ó¦·½³ĢŹ½ĪŖ_________________________________”£
(3)ČōZ”¢WČÜŅŗµÄpH¾łĪŖ5£¬ŌņĮ½ČÜŅŗÖŠÓÉĖ®µēĄė³öH+µÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ_________”£
(4)ŅŃÖŖ5.6 L(±ź×¼×“æö)ĘųĢå±ūŌŚ¼×ÖŠĶźČ«Č¼ÉշųöČČĮæĪŖ71.45 kJ£¬ŌņÄÜÕżČ·±ķŹ¾±ūČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ_____________________________________________”£
(1)O2+2H2O+4e-====4OH-
(2)FeCl3+3H2O![]() Fe(OH)3(½ŗĢå)+3HCl
Fe(OH)3(½ŗĢå)+3HCl
(3)1”Ć104
(4)H2(g)+![]() O2(g)====H2O(l)£»¦¤H=-285.8 kJ”¤mol-1
O2(g)====H2O(l)£»¦¤H=-285.8 kJ”¤mol-1
±¾ĢāÖŠÓŠŅ»Š©ĢŲÕ÷ŠŌÓļŃŌ£¬²Ō°×É«»šŃęŹĒH2ŌŚCl2ÖŠČ¼ÉÕµÄŃÕÉ«£¬¶”ŹĒ³£¼ū½šŹō£¬Č¼ÉÕĖłµĆ²śĪļĖ®ČÜŅŗÓÖĪŖ»ĘÉ«£¬ĖłŅŌÓ¦ĪŖFe3+ČÜŅŗŃÕÉ«£¬ČŻŅ×ĶĘ³öĪŖFeCl3”£(1)ĪŖĪüŃõøÆŹ“”£(2)ĪŖFe(OH)3½ŗĢåÖʱø”£(3)ZĪŖHCl£¬ĘäµēĄėĻŌĖįŠŌc(H+)=10-5£¬ÓÉĖ®µēĄėµÄcĖ®(H+)=cĖ®(OH-)=![]() =10-9£»¶ųFeCl3ĪŖĖ®½āĖįŠŌ£¬Ę䏵֏ĪŖĖ®µÄµēĄėcĖ®(H+)=10-5”£(4)Š“³ö·½³ĢŹ½ÓɶŌÓ¦±ČĒó·“Ó¦ČČ”£
=10-9£»¶ųFeCl3ĪŖĖ®½āĖįŠŌ£¬Ę䏵֏ĪŖĖ®µÄµēĄėcĖ®(H+)=10-5”£(4)Š“³ö·½³ĢŹ½ÓɶŌÓ¦±ČĒó·“Ó¦ČČ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

ÓÖÖŖ¢Ł¼×”¢ŅŅ”¢±ū¾łĪŖĒ°ČżÖÜĘŚŌŖĖŲµÄµ„ÖŹ£¬³£ĪĀĻĀ¾łĪŖĘųĢ¬£»¶”ŹĒČÕ³£Éś»īÖŠŅ»ÖÖ³£¼ūµÄ½šŹō”£
¢Ś³£ĪĀĻĀXŹĒĪŽÉ«ŅŗĢ壬YŹĒŗŚÉ«¹ĢĢ唣
¢Ū±ūŌŚŅŅÖŠČ¼ÉÕ·¢³ö²Ō°×É«»šŃę£¬¶”ŌŚŅŅÖŠČ¼ÉÕÉś³É×Ų»ĘÉ«µÄŃĢ£¬WµÄĖ®ČÜŅŗ³Ź»ĘÉ«”£
ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)¶”ŌŚ¼×ŗĶXĶ¬Ź±“ęŌŚµÄĢõ¼žĻĀ£¬æÉ·¢Éśµē»ÆѧøÆŹ“£¬Š“³öÕż¼«µÄµē¼«·“Ó¦Ź½£ŗ____________________________________________”£
(2)½«ÉŁĮæWµÄ±„ŗĶČÜŅŗµĪČėČȵÄXÖŠ£¬·“Ó¦·½³ĢŹ½ĪŖ_________________________________”£
(3)ČōZ”¢WČÜŅŗµÄpH¾łĪŖ5£¬ŌņĮ½ČÜŅŗÖŠÓÉĖ®µēĄė³öH+µÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ_________”£
(4)ŅŃÖŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

£Ø1£©¶”ÓėX·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________________________£»ŗģČȵĶ”ŗĶŅŅ·“Ó¦µÄĻÖĻóĪŖ___________________________________”£
£Ø2£©³£ĪĀĻĀ£¬ČōZ”¢WĮ½ČÜŅŗµÄpH¾łµČÓŚ5£¬ŌņÓÉĖ®µēĄė³öµÄH+µÄĪļÖŹµÄĮæÅضČĒ°ÕßÓėŗóÕߵıČÖµĪŖ______________________”£
£Ø3£©½«ÉŁĮæWµÄ±„ŗĶČÜŅŗµĪČė·ŠĢŚµÄXÖŠ£¬µĆµ½ŗģŗÖÉ«ŅŗĢ壬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________£¬“ĖŅŗĢå¾ßÓŠµÄŠŌÖŹŹĒ__________________£ØĢī×ÖÄø£©”£
a.¹āŹųĶعżøĆŅŗĢåŹ±ŠĪ³É¹āĮĮµÄ”°ĶØĀ·”±
b.²åČėµē¼«ĶعżÖ±Į÷µēŗó£¬ÓŠŅ»¼«ø½½üŅŗĢåŃÕÉ«¼ÓÉī
c.ĻņøĆŅŗĢåÖŠ¼ÓČėĻõĖįŅųČÜŅŗ£¬ĪŽ³Įµķ²śÉś
d.½«øĆŅŗĢå¼ÓČČ”¢ÕōøÉ”¢×ĘÉÕŗó£¬ÓŠŃõ»ÆĪļÉś³É
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µ„ÖŹŌŚµćČ¼Ģõ¼žĻĀ·“Ӧɜ³ÉX”¢Y”¢Z”¢WĖÄÖÖ»ÆŗĻĪļ£¬Ęä×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾”£
ÓÖÖŖ¢Ł¼×”¢ŅŅ”¢±ū¾łĪŖĒ°ČżÖÜĘŚŌŖĖŲµÄµ„ÖŹ£¬³£ĪĀĻĀ¾łĪŖĘųĢ¬£»¶”ŹĒČÕ³£Éś»īÖŠŅ»ÖÖ³£¼ūµÄ½šŹō”£
¢Ś³£ĪĀĻĀXŹĒĪŽÉ«ŅŗĢ壬YŹĒŗŚÉ«¹ĢĢ唣
¢Ū±ūŌŚŅŅÖŠČ¼ÉÕ·¢³ö²Ō°×É«»šŃę£¬¶”ŌŚŅŅÖŠČ¼ÉÕÉś³É×Ų»ĘÉ«µÄŃĢ£¬WµÄĖ®ČÜŅŗ³Ź»ĘÉ«”£
ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©¶”ŌŚ¼×ŗĶXĶ¬Ź±“ęŌŚµÄĢõ¼žĻĀ£¬æÉ·¢Éśµē»ÆѧøÆŹ“£¬Š“³öÕż¼«µÄµē¼«·“Ó¦Ź½£ŗ____
______________________________________________£»
£Ø2£©½«ÉŁĮæWµÄ±„ŗĶČÜŅŗµĪČėČȵÄXÖŠ£¬·“Ó¦·½³ĢŹ½ĪŖ_________________________£»
£Ø3£©ČōZ”¢WČÜŅŗµÄpH¾łĪŖ5£¬ŌņĮ½ČÜŅŗÖŠÓÉĖ®µēĄė³öH+µÄĪļÖŹµÄĮæÅضČÖ®±ČĪŖ_________£»
£Ø4£©ŅŃÖŖ5.6 L£Ø±ź×¼×“æö£©ĘųĢå±ūŌŚ¼×ÖŠĶźČ«Č¼ÉշųöČČĮæĪŖ71.45 kJ£¬ŌņÄÜÕżČ·±ķŹ¾±ūČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ_____________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ¶ĢÖÜĘŚÖŠµÄ5ÖÖŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£AŌŚÖÜĘŚ±ķÖŠŌ×Ó°ė¾¶×īŠ”£¬BŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒÄŚ²ćµē×ÓŹżµÄ2±¶£¬DŌŖĖŲÓėAŌŖĖŲĶ¬Ö÷×唣EŌŖĖŲÓėCŌŖĖŲĶ¬Ö÷×壻EµÄµ„ÖŹĪŖ»ĘÉ«¾§Ģ壬Ņ×ČÜÓŚ¶žĮņ»ÆĢ¼”£
¢Ł»³öAµÄŅõĄė×ӵĽį¹¹Ź¾ŅāĶ¼ ”£
¢ŚCµÄµ„ÖŹŗĶDµÄµ„ÖŹŌŚµćČ¼Ģõ¼žĻĀ·“Ӧɜ³É»ÆŗĻĪļX£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
”£½«²śĪļ¼ÓČėµĪÓŠ·ÓĢŖµÄĖ®ÖŠ£¬¹Ū²ģµ½µÄĻÖĻóĪŖ
ӣ
¢Ū½«9gBµ„ÖŹŌŚ×ćĮæµÄCµ„ÖŹÖŠČ¼ÉÕ£¬ĖłµĆĘųĢåĶØČė1L1.0mol”¤L-1NaOHČÜŅŗÖŠ£¬ĶźČ«ĪüŹÕŗó£¬ČÜŅŗÖŠ“ęŌŚµÄĄė×Ó°“ÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņŹĒ
ӣ
¢Ü½«EµÄµ„ÖŹŌŚ×ćĮæµÄCµÄµ„ÖŹÖŠČ¼ÉÕ£¬ĖłµĆÖ÷ŅŖĘųĢåĪŖY”£½«YĶØČėXÖŠ£¬XÓėYæÉÄÜ·¢ÉśµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ÓŠ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com