
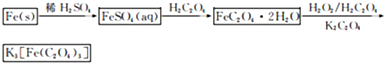
K3[Fe��C2O4��3]��3H2O[�����������������ؾ���]������ˮ���������Ҵ�������Ϊ�л���Ӧ�Ĵ�����ʵ���ҿ�����мΪԭ���Ʊ�����ط�Ӧ�������¡���ش��������⣺
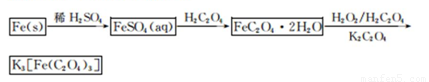
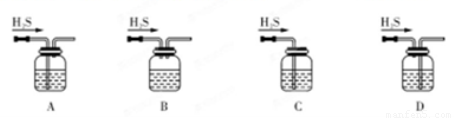
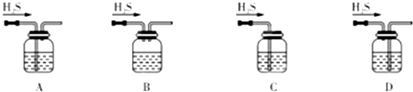
��1����м�г�����Ԫ�أ�������Ʊ�FeSO4ʱ������ж���H2S���壬�������������������Һ���ա���������װ����ȷ����??????????????? ������ţ���
��2���ڵõ���FeSO4��Һ�������������H2 SO4�ữ��Ŀ����?????????????????? ���õ�K3[Fe(C2O4)3]��Һ�����Ҵ���Ŀ����??????????????????????????? ��
��3�������������ᾧˮ��ͨ�������������ⶨ����Ҫ�����У��������������ں������ѽᾧˮ������ȴ�������������ظ������������أ������㡣
��������Ŀ����????????????????????????????????????????????????????????????????? ��
��4��C2O �ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
�ɱ�����KMnO4��Һ����ΪCO2���壬��ʵ�������K3[Fe��C2O3��3]��3H2O�����ⶨ����KMnO4����Һ�ζ���
��д���ζ������з�����Ӧ�����ӷ���ʽ???????????????????????????????????????????? ��
�����еζ�������ʹ�ζ����ƫ�ߵ���????????????? ������ţ���
A���ζ���������ˮϴ�Ӻ�����װ���Һ
B����ƿ��װ����Һǰδ�ô���Һ��ϴ
C���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ���������ʧ
D����ȡ��Һ���ʱ���ζ�ǰ���Ӷ������ζ����Ӷ���
��ȡ����10.0 g���100 mL��Һ������ȡ��20 mL����ƿ�У���Ũ��Ϊ0��1mol��L��l������KMnO4��Һ�ζ����ﵽ�ζ��յ�ʱ��������KMnO4��Һ24��00mL���������K3 [Fe��C2O4��3]��3H2O����������Ϊ???? [��֪Ħ������M{ K3[Fe��C2O4��3]��3H2O��=491 g��mol��l]��
��1��A��2������Fe2����ˮ��? ������������������������������������3��ȷ���ᾧˮȫ��ʧȥ��
��4����5C2O42��+2MnO4��+16H��=2Mn2��+10CO2��+8H2O����AC? ��49.1%
��������
�������������1����װ��������������������Һ�Ӵ�����Ӷ�ʹ�������ս���ȫ���Ҹ�װ������ѹ���ȶ�����������ȫ���⣬��A��ȷ���������������ƽӴ������С���������ղ���ȫ����B����û������װ�ã����¸�װ������ѹ�����������ȫ�¹ʣ���C����װ����Ӧ��ѭ�������̳�����ԭ������D��������ѡA��
��2������������ˮ���������ѹ�����ԣ�����ϡ������������������ˮ�⣻˫��ˮ���ȶ����¶ȸ�ʱ��˫��ˮ�ֽ⣬Ϊ��ֹ˫��ˮ�ֽ⣬�¶�Ӧ��Щ��������������ԭ��֪�����������������Ҵ����ܽ��С�����Կ������Ҵ�ʹ������������������
��3����������ˮ��Ϊ��ֹ��ˮ�������Ҫ�ڸ������н��У�����ݵ��Ǽ��龧���еĽᾧˮ�Ƿ���ȫ��ʧȥ��
��4���ٻ��ϼ�C��+3��+4���ı�����4-3����2=2��Mn��+7��+2���ı�����7-2����1=5�����ݻ��ϼ�����������ȣ�������C2O42��ǰ��5��MnO4��ǰ��2������C��Mnԭ���غ㣬�ֱ���CO2��Mn2��ǰ��10��2�����ݵ���غ㣬��H��ǰ��16�����������ӷ���ʽ���ߵ�H���������ˮǰ����8�����������ӷ���ʽ���ߵ���ԭ����ȣ��õ����ӷ���ʽΪ5C2O42��+2MnO4��+16H��=2Mn2��+10CO2��+8H2O��
��A���ζ���û���ñ�Һ��ϴ��Ũ�ȼ�С�����ĵı�Һ�����ʵ�������ⶨ���ƫ�ߣ�
B����ƿδ��ϴ����ȡ����Һ����û�䣬�ʲ�Ӱ��V�����������Եζ������Ӱ��
C���������ݵ���������˱�Һ���ĵ������ʹ�ñ�Һ�����ʵ��������ⶨ���ƫ�ߣ�
D����ȡ��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС���ⶨ���ƫ�ͣ�
��ѡAC��
��? 5K3[Fe(C2O4)3]��3H2O????????? ~????????????? 15C2O42��?????????? ~???????????? 6MnO4��
???????????? 5��491g?????????????????????????????????????????????? 6mol
x???????????????????????????????????????? 24.00mL��10��3��0.1mol��L��l
x ��0.982
K3 [Fe��C2O4��3]��3H2O����������0.982/2��100%��49.1%
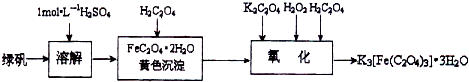
���㣺�����˻�ѧʵ���ۺ�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| O | - 4 |
| O | 2- 4 |

 ͼ��ʾװ�õļ�ѹ������ĸҺ���룮
ͼ��ʾװ�õļ�ѹ������ĸҺ���룮| O | 2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com