

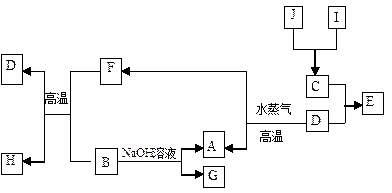
 (1)Š“³öĻĀĮŠĪļÖŹµÄĆū³ĘA £»G ”£
(1)Š“³öĻĀĮŠĪļÖŹµÄĆū³ĘA £»G ”£ (2)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
(2)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ¢ŁB+F”śD+H£ŗ £»
¢ŁB+F”śD+H£ŗ £» ¢ŚD”śA+F£ŗ £»
¢ŚD”śA+F£ŗ £» ¢ŪB”śA+G£ŗ ”£
¢ŪB”śA+G£ŗ ”£ (3)½«ŹŹĮæJ¼ÓČėĖį»ÆµÄH2O2µÄČÜŅŗÖŠ£¬JČܽāÉś³ÉĖüµÄ+2¼ŪĄė×Ó£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ
(3)½«ŹŹĮæJ¼ÓČėĖį»ÆµÄH2O2µÄČÜŅŗÖŠ£¬JČܽāÉś³ÉĖüµÄ+2¼ŪĄė×Ó£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ  ”£
ӣ
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
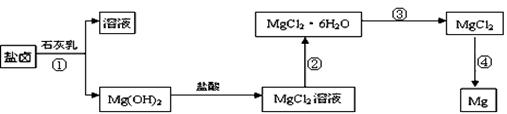
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
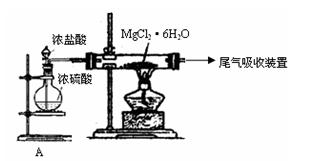
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
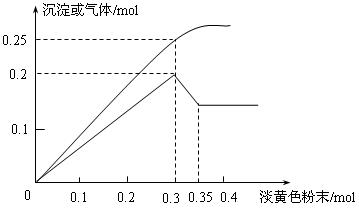
²éæ““š°øŗĶ½āĪö>>
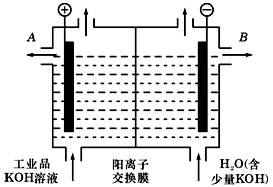
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ś¢Ü | B£®¢Ś¢Ū | C£®¢Ł¢Ü | D£®Ö»ÓŠ¢Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĀĮŹĒ»¹Ō¼Į£¬Ę仹Ō²śĪļŹĒAl(OH)3 |
| B£®NaOHŹĒŃõ»Æ¼Į£¬Ę仹Ō²śĪļŹĒH2 |
| C£®ĀĮŹĒ»¹Ō¼Į£¬H2OŗĶNaOH¶¼ŹĒŃõ»Æ¼Į |
| D£®H2OŹĒŃõ»Æ¼Į£¬Al±»Ńõ»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Al2O3 | B£®Al(OH)3 | C£®AlCl3 | D£®Fe(NO3)3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Ńõ»ÆĀĮŗĶŃĪĖį | B£®ĀĮŗĶĀČĘų |
| C£®Ńõ»ÆĀĮŗĶÉÕ¼īČÜŅŗ | D£®ĒāŃõ»ÆĀĮŗĶŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com