
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
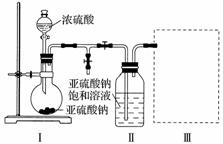
(1)װ�â��в�������Ļ�ѧ����ʽΪ_________________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����___________________________��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ__________(�����)��

ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO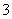 �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH
b������Ba(OH)2��Һ
c����������
d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)����Na2S2O5�����ڿ������ѱ�������ʵ�鷽����__________��
�𰸡�(1)Na2SO3��H2SO4(Ũ)===Na2SO4��SO2����H2O(��Na2SO3��2H2SO4(Ũ)===2NaHSO4��SO2����H2O)
(2)���ˡ�(3)d��(4)ae
(5)ȡ����Na2S2O5�������Թ��У�������ˮ�ܽ⣬�μ��������ᣬ���ٵ����Ȼ�����Һ���а�ɫ��������
������(1)װ�â��з�������ʵ�����Ʊ�SO2�ķ�Ӧ�����õ�ԭ����ǿ���Ʊ����ᣬ�ʻ�ѧ����ʽΪNa2SO3��H2SO4(Ũ)===Na2SO4��SO2����H2O��
(2)�����������ƾ������Һ�з������Ӧ�ò�ȡ���˵ķ�����
(3)ʵ�������β����Ҫ��SO2���壬aװ�����ܱյĻ�����SO2ͨ����ȥ��bװ����ʳ��ˮ����SO2��Ч������d�ã�������������cװ��ŨH2SO4������SO2��SO2��һ����������������NaOH��Һ������ã���dװ�û�������������
(4)HSO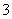 �������룺HSO
�������룺HSO H����SO
H����SO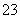 ��ͬʱ�ᷢ��ˮ�⣺HSO
��ͬʱ�ᷢ��ˮ�⣺HSO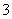 ��H2OH2SO3��OH������HSO
��H2OH2SO3��OH������HSO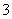 �ĵ������HSO
�ĵ������HSO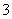 ��ˮ�⣬����Һ�����ԣ��ʴ�a��e��ȷ��
��ˮ�⣬����Һ�����ԣ��ʴ�a��e��ȷ��
(5)Na2S2O5��SԪ�صĻ��ϼ�Ϊ��4�ۣ�����ڿ������ױ�����Ϊ��6�۵�SO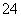 ����˱����ת��ΪSO
����˱����ת��ΪSO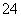 �ļ��飬��ȡ���������ȼ������ữ���ų��������ӵĸ��ţ��ټ�BaCl2��Һ�����Ƿ��а�ɫ�����������ɡ�
�ļ��飬��ȡ���������ȼ������ữ���ų��������ӵĸ��ţ��ټ�BaCl2��Һ�����Ƿ��а�ɫ�����������ɡ�


 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ס��ҡ��������ĸ��ձ��ڷֱ����0.1 mol���ơ������ơ��������ƺ��������ƣ�Ȼ�������100 mLˮ�����裬ʹ������ȫ�ܽ⣬��ס��ҡ���������Һ�����ʵ�����������С˳����(����)
A���ף��ң������� B�������ף��ң���
C���ף������ң��� D�������ף��ң���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ���ۺ����ÿ����Ʊ�����þ�����������£�

(1)��ˮ��þҪ�õ���̲�ϵı��ǣ�����������________________________________�����Ǿ������йر仯�Ļ�ѧ����ʽ��____________________��
(2)д����MgCl2�õ�����þ�ķ�Ӧ����ʽ________________________________________________________________________
________________________________________________________________________��
(3)Mg(OH)2�����л���Ca(OH)2Ӧ������ȥ��(д��ʵ�鲽��)________________________________________________________________________��
(4)�Ӿ���Ч��Ƕȿ����û������ij�ַӦѡ����________________________________________________________________________
________________________________________________________________________��
(5)ʵ�����н������Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵����������������õ���������Ŀ�ġ�
�ܽ�ʱ��________________________________________________________________________��
����ʱ��________________________________________________________________________��
����ʱ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ÿ��ѡ���е��������ʶ��ܷ�Ӧ�����ܷų�ͬһ���������(����)
A��ͭ��ϡ���ᣬͭ��Ũ����
B������ϡ���ᣬ����Ũ����
C��������ϡ���ᣬ����������ϡ����
D��������ϡ���ᣬ������ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������(Na2S2O3)�����������Լ�������Ļ�ԭ���������ȡ������ֽ⡣��ҵ�Ͽ��÷�Ӧ��2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2 �Ƶá�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ���ش��������⣺

(1)b�з�Ӧ�����ӷ���ʽΪ__________________________________��
c���Լ�Ϊ____________��
(2)��Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������____________��
(3)d�е��Լ�Ϊ______________��
(4)ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��
________________________________________________________________________
___________________________________________________(�����)��
(5)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ��SO2���ܹ�����ԭ����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵ����ͬһԭ�����͵���(����)
A��Ũ�����Ũ���᳤�ڱ�¶�ڿ�����Ũ�Ƚ���
B��SO2��FeSO4��Һʹ���Ը�����ص���ɫ��ȥ
C��Ư�ۺ�ˮ�������ڱ�¶�ڿ����б���
D������������Һ���Ȼ�����Һ�ڿ��������ɲ��ܵõ���Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ(���ֲ���δ�г�)��A��һ�ֽ������ʣ�D��һ�ַǽ������嵥�ʡ�

��ش��������⣺
(1)A��C�Ļ�ѧʽ�ֱ�ΪA__________��C__________��
(2)F��Ũ��Һ��A��Ӧ�����У�F���ֵ����������з�Ӧ��H2SO4���ֵ�������ȫ��ͬ����________(����ĸ)��
A��C��2H2SO4(Ũ)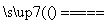 CO2����2SO2����2H2O
CO2����2SO2����2H2O
B��Fe��H2SO4===FeSO4��H2��
C��Cu��2H2SO4(Ũ)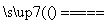 CuSO4��SO2����2H2O
CuSO4��SO2����2H2O
D��FeO��H2SO4===FeSO4��H2O
(3)д����ӦE��H2O2����F�Ļ�ѧ����ʽ��_______________________________________��
(4)����ӦF��D����Eת�Ƶ�����Ϊ6.02��1023��������D������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ�Ӻ�������Ų�ʽΪ[Ar] 3d54s2��Ԫ���ǣ� ��
A. Cr B.ds ��Ԫ�� C.��A��Ԫ�� D. ��B��Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
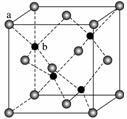
ZnS��ӫ���塢�����ϡ�Ϳ�ϡ����ϵ���ҵ��Ӧ�ù㷺������ZnS����ṹ��ͼ��ʾ���侧���߳�Ϊ540.0 pm���ܶ�Ϊ______g·cm��3(��ʽ������)��aλ��S2����bλ��Zn2��֮��ľ���Ϊ______pm(��ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com