



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B����ȡ��ˮ�еĵⵥ��ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
| C����PH��ֽ�ⶨij��ɫ��Һ��PHʱ��Ӧ��PH��ֽ������Һ�У��۲�����ɫ�仯��������ɫ���Ƚ� |
| D����������У��¶ȼ�Ӧ�÷���������ƿ�ڵ�Һ���У��Բ���Һ����¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij�������ϡ����,������ɫ��ʹ����ʯ��ˮ����ǵ�����,֤���ù���һ����CO32- |
| B�������ռ���Һ���Ƿ�Cl-���ȼ�ϡ���ᣬ�ټ�AgNO3��Һ��������ɫ���� |
C��ij��Һ�еμ�BaCl2��Һ���ټ�ϡHNO3��������ɫ������ԭ��Һ��һ����SO |
| D��ij��Һ�е���KSCN��Һ�����Ա仯���ټ�����ˮ�ʺ�ɫ��ԭ��Һ��һ����Fe2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������KOH����ʱ��ֱ����ֽ������ |
| B��KOH�����ܽ��δ��ȫ��ȴ��ת��������ƿ�У� |
| C������ʱ����������ƿ���ߣ� |
| D��������Һǰ������ƿδ��ȫ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
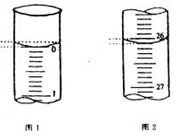
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ����ɫ��Һ ���ɫ���� ���ɫ���� | ˵��ԭ��Һ��һ������FeCl3 |
| B | Br2 FeBr3 FeBr3 KBr KBr | �Ʊ�����KBr��Һ |
| C | ��ɫ��Һ ��ɫ�ʻ�ɫ ��ɫ�ʻ�ɫ | ˵��ԭ��Һ��һ��������Ԫ�� |
| D | H3PO3+2NaOH(����)=Na2HPO3+2H2O | H3PO3������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
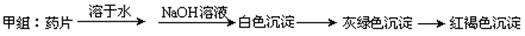 ����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������
����ͬѧ������Ƶķ������ʵ�飬���ź���������û�еõ�Ԥ�ڵ�ʵ������ 
 ����ʵ����֤��________________________________
����ʵ����֤��________________________________| ��� | V��KMnO4���� | V��KMnO4���� | V��KMnO4�� |
| 1 | 2.24mL | 14.25mL | 12.01mL |
| 2 | 0.30mL | 12.72mL | 12.42mL |
| 3 | 0.50mL | 12.53 | 12.03mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
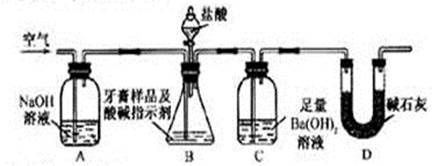
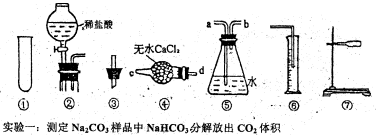
 12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á�
12�֣���̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����ֽ��ҽҩ�����������д���Ӧ�á� 2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�
2H2O2 ��2H2O+ O2��.Ϊ�ⶨ�ѱ��ʵĹ�̼����(��̼����)�Ĵ��ȣ������ͼ��ʾ��ʵ�飺QΪ���������õĵ��Ե������뷴Ӧ��������ﷴӦ������ȡһ��������Ʒ�������������̷������У���ͼ��װ��ʵ��װ�ã���Һ©���Ļ�������ϡH2SO4���������С�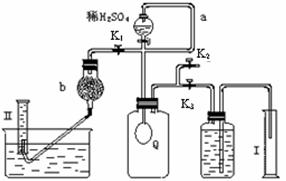
 ____________
____________ _____��
_____��| A������������Q�͵������У�δȫ��������Ͳ�� |
B����Ͳ�����ʱ ����ͲҺ�����ˮ��Һ�� ����ͲҺ�����ˮ��Һ�� |
| C���Ҳ���Ͳ��ʹ�Һ����ƿ���ӵ����ڵ�Һ��û�м������x |
| D�����������ֵx��yû�п۳��μӵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com