
·ÖĪö £Ø1£©ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
£Ø2£©øł¾ŻĢģĘ½µÄÕżČ·µÄŹ¹ÓĆ·½·Ø½ā“š£»
£Ø3£©øł¾ŻŹµŃé²Ł×÷µÄ²½ÖčŅŌČ·¶ØČÜŅŗÅäÖĘĖłŠčŅĒĘ÷£»
£Ø4£©·ÖĪö²Ł×÷¶ŌČÜŅŗĪļÖŹµÄĮ攢ČÜŅŗĢå»żµÄÓ°Ļģ£¬øł¾Żc=$\frac{n}{V}$·ÖĪö²Ł×÷¶ŌĖłÅäČÜŅŗÅØ¶ČµÄÓ°Ļģ£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ500mL0.1mol/LNa2CO3ČÜŅŗ£¬ŠčŅŖŹ®Ė®Ģ¼ĖįÄĘ¾§ĢåµÄÖŹĮæm=0.1mol/L”Į0.5L”Į286g/mol=14.3g£»
¹Ź“š°øĪŖ£ŗ14.3£»
£Ø2£©ÓĆĢģĘ½³ĘĮæĪļĢåŹ±µÄÕżČ·²Ł×÷²½ÖčŹĒ£ŗĻČ½«ÓĪĀėŅĘÖĮæĢ¶Č³ßµÄĮćæĢ¶Č“¦²¢µ÷Įćµć£¬Č»ŗóĻČ³ĘĮææյĊ”ÉÕ±µÄÖŹĮ棬¼ĒĀ¼³ĘĮæµÄ½į¹ū£¬½«Ģ¼ĖįÄĘ¾§Ģå·ÅČėŠ”ÉÕ±ÖŠ³ĘĮ棬¼ĒĀ¼³ĘĮæµÄ½į¹ū£¬½«ķĄĀė·Å»ŲķĄĀėŗŠÄŚ£¬×īŗó½«ÓĪĀėŅĘÖĮæĢ¶Č³ßµÄĮćæĢ¶Č“¦£¬
¹Ź“š°øĪŖ£ŗADFCFE£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄŅ»°ć²Ł×÷²½ÖčĪŖ£ŗ³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬Ņ»°ćÓĆĶŠÅĢĢģĘ½³ĘĮ棬ÓĆŅ©³×Č”Ņ©Ę·£¬ŌŚÉÕ±ÖŠČܽā£¬²¢ÓĆ²£Į§°ō½Į°č£¬ĄäČ“ŗó×ŖŅʵ½500mlČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬Ļ“µÓ²¢½«øĆĻ“µÓŅŗŅĘČėČŻĮæĘæÖŠ£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬ĖłŠčŅŖµÄŅĒĘ÷ĪŖ£ŗÉÕ±”¢ĶŠÅĢĢģĘ½”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü”¢Ņ©³×£¬
¹Ź“š°øĪŖ£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
£Ø4£©A”¢¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ»
B”¢Ć»ÓŠ½«Ļ“µÓŅŗ×ŖČėČŻĮæĘ棬µ¼ÖĀ²æ·ÖČÜÖŹĖšŹ§£¬ČÜÖŹµÄĪļÖŹµÄĪļÖŹµÄĮæĘ«Š”ČÜŅŗÅضČĘ«µĶ£»
C”¢ČŻĮæĘæÄŚ±Śø½ÓŠĖ®Öé¶ųĪ“øÉŌļ“¦Ąķ²»±ä¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£»
¹Ź“š°øĪŖ£ŗĘ«øߣ»Ę«µĶ£»²»±ä£»
µćĘĄ ±¾Ģāæ¼²éŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ²Ł×÷¼°Īó²ī·ÖĪöµČ£¬ÄѶČÖŠµČ£¬×¢Ņāøł¾Żc=$\frac{n}{V}$Ąķ½āČÜŅŗµÄŌĄķÓėĪó²ī·ÖĪö£¬×¢ŅāĶŠÅĢĢģĘ½Ź¹ÓƵķ½·Ø£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 150mL””0.5””mol/L””MgCl2ČÜŅŗ | B£® | 100mL””1.0mol/L””HClČÜŅŗ | ||
| C£® | 250mL””1.5mol/L””NaClČÜŅŗ | D£® | 300””mL””0.25mol/L””CaCl2ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
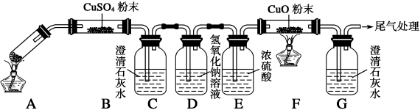
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ČēĶ¼ŹĒÄ³Ń§Š£ŹµŃéŹŅ“Ó»ÆѧŹŌ¼ĮÉĢµźĀņ»ŲµÄÅØĮņĖįŹŌ¼Į±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ŹĒÄ³Ń§Š£ŹµŃéŹŅ“Ó»ÆѧŹŌ¼ĮÉĢµźĀņ»ŲµÄÅØĮņĖįŹŌ¼Į±źĒ©ÉĻµÄ²æ·ÖÄŚČŻ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
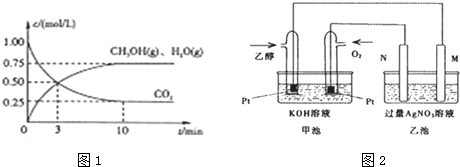
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
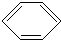 +Cl2
+Cl2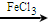
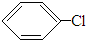 +HCl£¬·“Ó¦ĄąŠĶČ”“ś·“Ó¦£®
+HCl£¬·“Ó¦ĄąŠĶČ”“ś·“Ó¦£®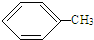 +3HO-NO2
+3HO-NO2
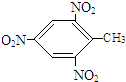 +3H2O£¬·“Ó¦ĄąŠĶČ”“ś·“Ó¦£®
+3H2O£¬·“Ó¦ĄąŠĶČ”“ś·“Ó¦£® +3H2
+3H2
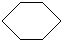 £¬·“Ó¦ĄąŠĶ¼Ó³É·“Ó¦£®
£¬·“Ó¦ĄąŠĶ¼Ó³É·“Ó¦£® +Br2
+Br2
 +HBr£¬·“Ó¦ĄąŠĶČ”“ś£®
+HBr£¬·“Ó¦ĄąŠĶČ”“ś£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
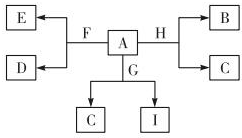 ČēĶ¼ŹĒ֊ѧ»ÆŃ§Ä³Š©ĪļÖŹÖ®¼äµÄĻą»„×Ŗ»Æ¹ŲĻµ£¬ŅŃÖŖAŹĒÖÖ³£¼ūµÄŅŗĢ¬»ÆŗĻĪļ£¬C”¢D”¢G”¢H¾łĪŖµ„ÖŹ£¬G”¢HŠĪ³ÉµÄŗĻ½šŹĒµ±Ē°Ź¹ÓĆĮæ×ī“óµÄŅ»ÖÖŗĻ½š£¬BŹĒŗŚÉ«¹ĢĢ壮
ČēĶ¼ŹĒ֊ѧ»ÆŃ§Ä³Š©ĪļÖŹÖ®¼äµÄĻą»„×Ŗ»Æ¹ŲĻµ£¬ŅŃÖŖAŹĒÖÖ³£¼ūµÄŅŗĢ¬»ÆŗĻĪļ£¬C”¢D”¢G”¢H¾łĪŖµ„ÖŹ£¬G”¢HŠĪ³ÉµÄŗĻ½šŹĒµ±Ē°Ź¹ÓĆĮæ×ī“óµÄŅ»ÖÖŗĻ½š£¬BŹĒŗŚÉ«¹ĢĢ壮²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĻõĖįŅų | B£® | ĮņĖįĢś | C£® | ĮņĖįĶ | D£® | ĀČ»ÆĆ¾ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬71g Cl2Ėłŗ¬Ō×ÓŹżĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬22.4Lŗ¤ĘųÖŠŗ¬ÓŠµÄŌ×ÓŹżĪŖ2NA | |
| C£® | ŌŚ·Ē±ź×¼×“æöĻĀ£¬1molČĪŗĪĘųĢåµÄĢå»ż²»æÉÄÜŹĒ22.4L | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬32g O2ŗĶO3»ģŗĻĘųĢåÖŠŗ¬ÓŠŌ×ÓŹżĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com