
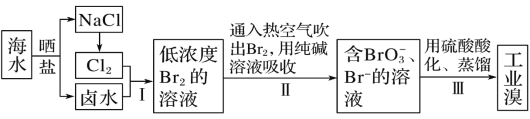
����Ŀ����֪��ˮ�е��庬��ԼΪ 65 mg��L-1���Ӻ�ˮ����ȡ��Ĺ�ҵ�������£�
(1)д�����������ӷ���ʽ________________��
(2)�����ͨ���ȿ������� Br2�����������_________________(�����)��
A.������ B.��ԭ�� C.�ӷ��� D.��ʴ��
(3)��������漰�����ӷ�Ӧ���£����ں�����������ȷ�Ļ�ѧ��������______Br2+______CO32-=______BrO3-+______Br-+______CO2��
(4)���������д�����������Ҳ�������ö��������ˮ��Һ���գ���������������Ȼ�����������������������ˮ��Һ��Ӧ�����ӷ���ʽΪ_______________��
(5)ʵ���ҷ����廹��������ȡ�������п������������ȡ������________________(����ĸ)��
A.�Ҵ� B.���Ȼ�̼ C.���� D.��
���𰸡�Cl2+2Br-===2Cl-+Br2 C 3 3 1 5 3 SO2+Br2+2H2O�T2Br-+SO42-+4H+ BD
��������
���ݹ�������ͼ����ˮɹ�κ�õ�±ˮ��NaCl��ͨ�����NaCl�õ�Cl2��Cl2��±ˮ�е������������õ��ϵ�Ũ�ȵ�Br2��Һ������Br2�ӷ������ʣ�������ͨ���ȿ�������Br2���ô�����Һ���գ��õ�����BrO3-��Br-����Һ����ͨ�������ữ�������һϵ�в����õ���ҵ�壬����̵���ҪĿ���Ǹ����壬����Ũ�ȵ�Br2��Һ��ɸ�Ũ�ȵ�Br2��Һ�����������ᴿ�ijɱ����ݴ˷���������⡣
(1)�������������������ΪCl2��±ˮ�е������������õ�Br2����Ӧ�����ӷ���ʽΪCl2+2Br-===2Cl-+Br2���ʴ�Ϊ��Cl2+2Br-===2Cl-+Br2��
(2)�������������Br2�ӷ������ʣ�������ͨ���ȿ�������Br2���ô�����Һ���գ��ʴ�Ϊ��C��
(3)����������ԭ��Ӧ���ɣ�ת�Ƶ����غ㡢ԭ���غ�Է���ʽ������ƽ�У�3Br2+3CO32-=BrO3-+5Br-+3CO2�����ʴ�Ϊ��3��3��1��5��3��
(4) ������������ˮ��Һ����������ԭ��Ӧ��������������ᣬ�����ӷ�Ӧ����ʽΪSO2+Br2+2H2O�T2Br-+SO42-+4H+���ʴ�Ϊ��SO2+Br2+2H2O�T2Br-+SO42-+4H+��
(5)A��Ҵ�����ˮ���ʲ�ѡA��
B����Ȼ�̼������ˮ�������巴Ӧ�����������Ȼ�̼���ܽ�ȴ�����ˮ�е��ܽ�ȣ���ѡB��
C�������Һ��ˮ���ܣ��ʲ�ѡC��
D���������ˮ�������巴Ӧ�������ڱ����ܽ�ȴ�����ˮ�е��ܽ�ȣ���ѡD��
���ϣ��������������ȡ���������Ȼ�̼�ͱ����ʴ�Ϊ��BD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��H ��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���
Ԫ�� | �����Ϣ |
A | ԭ�Ӻ����� 6 �ֲ�ͬ�˶�״̬�ĵ��� |
C | ��̬ԭ���� s ����������p ����������� |
D | ԭ�Ӱ뾶��ͬ����Ԫ������� |
E | ��̬ԭ�����������Ų�ʽΪ 3s23p1 |
F | ��̬ԭ�ӵ������ p ������������ӵ������������������ӵ��� �������෴ |
G | ��̬ԭ�Ӻ����� 7 ���ܼ���������ߵ��ܼ����� 6 ������ |
H | ���ҹ�ʹ������ĺϽ��е�����Ҫ����Ԫ�� |
(1) A Ԫ�ص�������_______________��A Ԫ�غ� F Ԫ���γɵ��������ķ��ӹ���Ϊ_______________���÷�����_______________���ӡ������������������Ǽ�������
(2) B Ԫ���γɵĵ��ʷ�����������������Ŀ֮��Ϊ_______________��
(3) G Ԫ�صĵͼ������ӵ����ӽṹʾ��ͼ��_______________��
(4) G �ĸ������ӵ���Һ�� H ���ʷ�Ӧ�����ӷ���ʽΪ___________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йذ����ӵ�����NA�������в���ȷ���ǣ� ��
A.1mol NaHSO4������������������ĿΪNA
B.���³�ѹ�£�92 g��NO2��N2O4������庬�е�ԭ����Ϊ6NA
C.��״���£�22.4L HF���е�����Ϊ8NA
D.60gʯӢ�����к��е�Si-O����ĿΪ4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������Ҫ�Ļ���ԭ�ϣ���������ԭ������������ȥ�ȼ��ȡ��ش��������⣺
(1)Ҫ����250mL0.5mol/L��Na2SO3��Һ����Ҫ��������ƽ��ȡNa2SO3��7H2O����________g��������Һ���õ��IJ��������У����������ձ�����Ͳ��_________________��
(2)���ƹ�������������ˮ����С���ȴ�����ʹ�ã��������ˮ��Ŀ����________��
(3)���в�����ʹ������ҺŨ��ƫ�ߵ���________��
A.��ѡ�õ������Ѿ�ʧȥ���ֽᾧˮ B.�������ƾ���������λ�÷ŷ���
C.ת����Һʱ���������ڿ̶����Ϸ� D.����ʱ��������ƿ�Ŀ̶���
E.ҡ�Ⱥ���Һ���ڿ̶��ߣ��ټ�������ˮʹҺ����͵���̶�������
(4)Na2SO3���ڿ��������ױ��������ʡ�����Na2SO3��Һ�Ƿ���ʵķ�����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���¶Ⱥ�ѹǿ��X+Y![]() 2Z��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Z���������������������ȷ���ǣ� ��
2Z��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Z���������������������ȷ���ǣ� ��
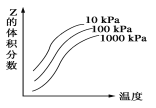
A.�������淴Ӧ������ӦΪ���ȷ�Ӧ
B.X��Y��ֻ��һ��Ϊ��̬��ZΪ��̬
C.X��Y��Z������
D.������Ӧ���淴Ӧ��H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ѧ�������ʴ���ת����ϵ��A+B��C+D+H2O������C�������࣬�����ƶϲ���ȷ����
A.��AΪ����ɫ���壬��D������Ư����
B.��AΪ��ɫ�������ʣ���Dһ���Ǻ���ɫ����
C.��AΪ�����Dһ����ʹ����ʯ��ˮ�����
D.��AΪ�Ȼ�泥���Dһ���Ǿ��д̼�����ζ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1,4-��������(![]() )��һ�ֳ������ܼ���������ͨ�����з����Ƶ���ͼ��ʾ������A��
)��һ�ֳ������ܼ���������ͨ�����з����Ƶ���ͼ��ʾ������A��
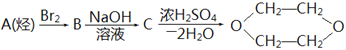
A. 1-��ϩ B. 1,3-����ϩ C. ��Ȳ D. ��ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л���AΪԭ�Ϻϳɾۺ���PMA�;�̼����PC��·�����£�
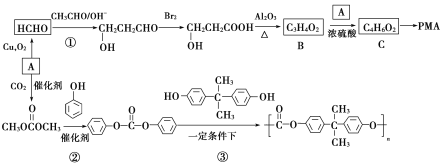
�ش��������⣺
(1)���й��ڸ߷��ӻ������˵������ȷ����________�������ţ�
a.����ϩ�����к���̼̼˫��
b.![]() �ĵ�����2-��Ȳ
�ĵ�����2-��Ȳ
c.������ë�Ͳ�˿����Ȼ��ά
d.CuSO4��Һ��ʹ�����ʱ���
(2)A�Ļ�ѧ������________��C�еĹ���������Ϊ_________��
(3)�ٵķ�Ӧ����Ϊ________���۵ķ�Ӧ����Ϊ________��
(4)��Ӧ�ڵĻ�ѧ����ʽΪ_____________________��
(5)B��ͬϵ��C5H8O2����____________��ͬ���칹�壨���������칹����д�����к˴Ź�������Ϊ���������ʵĽṹ��ʽ��______________��
(6)���������ϳ�·�ߣ���CH3CHOΪԭ��(���Լ���ѡ)������Ʊ�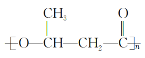 �ĺϳ�·��________________��
�ĺϳ�·��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
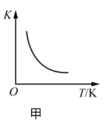
����Ŀ����������ͼʾ���ó��Ľ�����ȷ����
A.ͼ�ױ�ʾ��Ӧ![]() ��ƽ�ⳣ��K���¶ȵĹ�ϵ��˵���÷�Ӧ��
��ƽ�ⳣ��K���¶ȵĹ�ϵ��˵���÷�Ӧ��![]()
B.ͼ�ұ�ʾCu�����Ũ���ᷴӦ���ɵ�![]() ���������ʱ��ı仯��ϵ��˵���÷�Ӧ��
���������ʱ��ı仯��ϵ��˵���÷�Ӧ��![]() ʱ����ڷ�Ӧ�������
ʱ����ڷ�Ӧ�������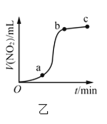
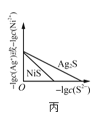
C.ͼ����ʾһ���¶���![]() ��NiS�ij����ܽ�ƽ�����ߣ�˵�����¶��£�
��NiS�ij����ܽ�ƽ�����ߣ�˵�����¶��£�![]() ʱ�����ߵ�
ʱ�����ߵ�![]() ���
���
D.ͼ����ʾ��ͬ�¶��£�![]() ��HF��
��HF��![]() ��Һ�ֱ��ˮϡ��ʱpH�ı仯���ߣ�˵����ˮϡ��ǰHF�ĵ���̶ȴ���
��Һ�ֱ��ˮϡ��ʱpH�ı仯���ߣ�˵����ˮϡ��ǰHF�ĵ���̶ȴ���![]() �ĵ���̶�
�ĵ���̶�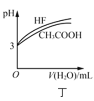
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com