
����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1������������һ����������(ֲ���������ڼ�)������ɽṹ���������ʼ��±���
����ʽ | �ṹ��ʽ | ��� | �۵� | �ܽ��� |
C12H10ClN3O |
| ��ɫ�ᾧ��ĩ | 170~172 �� | ������ˮ |
������֪������2-��-4-������������ᱽ����Ӧ�������������塣
![]()
��Ӧ�����У�ÿ����1 mol�������壬����____���Ҽ�������____���м���
��2�����ɽ���������ˮ�����γɵ�������Ƿ�����ɫ����d��������Ų��йء�һ��أ�d0��d10�Ų�����ɫ��d1��d9�Ų�����ɫ����Co(H2O)6]2���Էۺ�ɫ���ݴ��жϣ�Mn(H2O)6]2��_____(��ޡ����С�)��ɫ��
��3����Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ�������ᣨH3BO3����ˮ��Һ������ˮ��Ӧ����B(OH)4]��������һԪ��������ʣ���B(OH)4]����B��ԭ���ӻ�����Ϊ_________��
��4��Mg�ǵ�������Ԫ�أ������ڲ���Ԫ�ط�������۵���±���
������ | NaF | MgF2 | SiF4 |
�۵�/K | 1 266 | 1 534 | 183 |
���ͱ��з������۵�����ԭ��___________________________________��
��5���ҹ���ѧ�ҳɹ��ϳ������������嵪��������(N5)6(H3O)3(NH4)4Cl(��R ����)����X-���������û�����R �ľ���ṹ����ֲ��ṹ����ͼ��ʾ��

����ɻ�����R �������������ЦҼ��ĸ���֮��Ϊ____________�������ĺ������ӵ����幹��Ϊ____________��������ԭ�ӵ��ӻ����������____________��
�ڷ����еĴ�м����÷��ű�ʾ��mn������m ���������γɵĴ�м�ԭ������n ���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ��66����N5���еĴ�м�Ӧ��ʾΪ____________��
�����ʾ����ͼ�е����:________________________��
���𰸡� NA NA �� sp3 NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߡ���ΪMg2+�İ뾶С��Na+�İ뾶��������������࣬����MgF2�ľ����ܴ���NaF�ľ����ܣ���MgF2���۵����NaF 3:4(��4:3) ������ sp3 ��56 N-H������N�� O-H������N�� N-H������Cl
��������(1).�����ᱽ�����ѵ�N=C�к�1���м���2-��-4-����श��ѵ���1���Ҽ���(2).����������Ϣ��Mn(H2O)6]2����Mnԭ��d�ܼ��������ж������Ƿ�����ɫ��(3). ���ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��(4).��������۵�ߵ��뾧�������йأ����Ӿ�����۵�ϸߣ����Ӿ�����۵�ϵ���(5). ��.������ΪH3O����NH4����H3O���к���3���Ҽ���NH4���к���4���Ҽ�����. N5���л�ѧ������Ϊ5������6�������γɴ���������. ��ͼ��֪����ɱ�ʾΪN-H������N��O-H������N��N-H������Cl��
(1).�ڷ�Ӧ�����У������ᱽ�����ѵ�N=C�к�1���м���2-��-4-����श��ѵ���1���Ҽ�����ÿ����1 mol�������壬����NA���Ҽ���NA���м����ʴ�Ϊ��NA��NA��
(2). ���ɽ���������ˮ�����γɵ�������Ƿ�����ɫ����d��������Ų��й���һ��أ�d0��d10�Ų�����ɫ��d1��d9�Ų�����ɫ��Mn(H2O)6]2����Mnԭ��d�ܼ�������Ϊ5�����Ը���������ɫ���ʴ�Ϊ���У�
(3). B(OH)4]����B�ļ۲���Ӷ���Ϊ4+![]() =4������Bԭ�ӵ��ӻ�����Ϊsp3���ʴ�Ϊ��sp3��
=4������Bԭ�ӵ��ӻ�����Ϊsp3���ʴ�Ϊ��sp3��
(4). ���Ӿ�����۵�ϸߣ����Ӿ�����۵�ϵͣ�NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߣ�����ΪMg2+�İ뾶С��Na+�İ뾶����Mg2+�ĵ�����࣬����MgF2�ľ����ܴ���NaF������MaF2���۵����NaF���ʴ�Ϊ��NaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬����NaF��MgF2Զ��SiF4�۵�Ҫ�ߡ���ΪMg2+�İ뾶С��Na+�İ뾶��������������࣬����MgF2�ľ����ܴ���NaF�ľ����ܣ���MgF2���۵����NaF��
(5).��.��ͼ��֪��������ΪH3O����NH4����H3O���к���3���Ҽ���NH4���к���4���Ҽ�������R �������������ЦҼ��ĸ���֮��Ϊ3:4(��4:3)��H3O��������ԭ����O����۲���Ӷ���Ϊ3+![]() =3+1=4������Oԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ1������ռ乹��Ϊ�����Σ��ʴ�Ϊ��3:4(��4:3)����������sp3��
=3+1=4������Oԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ1������ռ乹��Ϊ�����Σ��ʴ�Ϊ��3:4(��4:3)����������sp3��
��.��ͼ��֪��N5���л�ѧ������Ϊ5������6�������γɴ����������÷�����56��ʾ���ʴ�Ϊ����56��
��.��ͼ��֪����ͼ�е�����ɱ�ʾΪN-H������N��O-H������N��N-H������Cl���ʴ�Ϊ��N-H������N��O-H������N��N-H������Cl��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������һ��������ɫ���������Ʊ�������صĹ����������£�
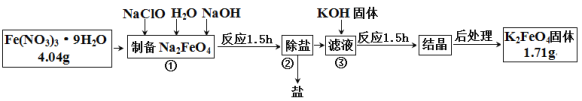
�ش��������⣺
��1���������NaClO��______���������������ԭ��������������
��2��������ѳ����γ�NaNO3 �⣬���� ____________���ѧʽ����
��3��������������ܽ�ȵIJ�ͬ���еIJ��������ܽ�ȣ�Na2FeO4 _____���>����<����K2FeO4��
��4������ʵ����K2FeO4�IJ���Ϊ__________________��
��5��ȡ����K2FeO4���Թ��У��������ữ����ס�Թܿڣ��۲쵽��Һ����ϸ��С���ݲ�������Һ��ɫ����ȥ������һ�������ǵ�ľ����ľ����ȼ��������Һ�м���KSCN��Һ����Һ��ΪѪ��ɫ���������²�������2.24L,��μӷ�Ӧ��FeO42- ��Ŀ_____________��K2FeO4��Ϊ��ˮ�����ŵ��������������ɱ�����__________________________________��
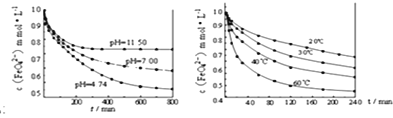
��6��ijͬѧ�����������ʵ��̽��������ص��ȶ��ԡ�
��ʵ��1��������K2FeO4����ֱ��ܽ���pHΪ4.74��7.00��11.50��ˮ��Һ�У����FeO42-Ũ��Ϊ1.0mmolL-1��1mmolL-1=10-3molL-1�������������ã������ͼ1��
��ʵ��2��������K2FeO4�ܽ���pH=4.74��ˮ��Һ�У����Ƴ�FeO42-Ũ��Ϊ1.0mmolL-1���������������ֱ����� 20�桢30�桢40���60��ĺ���ˮԡ�У������ͼ2��
��ʵ����ۣ�����ͼһ�����Եó��Ľ����ǣ�________________________________________
ͼ1 ͼ2
��7�������£�ijˮ��Һ����Fe3+,Cu2+,������ҺpH=10ʱ��������������������棬��֪���¶��£� Ksp��Fe(OH)3��= a, Ksp��Cu(OH)2��= b,����Һ��C(Fe3+)/C(Cu2+)=___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��֪���и��ֹ��ۼ��ļ��ܣ�C��H��a kJ�� mol��1��O��H��b kJ�� mol��1��O��O��c kJ��mol��1��C��O��d kJ��mol��1��д������������ȫȼ������CO2���������ˮ���Ȼ�ѧ����ʽ��_________________________________
(2)����е���Ҫȼ��������(Ҳ��Ϊ����)����Һ�����ʡ��˻��������Է�������Ϊ32�����У���N����������Ϊ87.5%������ΪHԪ�ء���
���µĻ�ѧʽΪ__________________����ṹʽΪ_____________________________
�ڴ�ȼ�ϵ���������N2O4���壬��ȼ��0.2 mol��Һ��ų�������Ϊ 400 kJ��ȼ�յIJ���Դ���������Ⱦ(���ɵ�ˮΪҺ̬)��������N2O4���巴Ӧ���Ȼ�ѧ����ʽΪ��__________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����80 ��ʱ����0.20 mol��N2O4�������1 L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
ʱ�� /(s) | 0 | 20 | 40 | 60 | 80 | 100 |
c(N2O4) / mol �� L��1 | 0.20 | c1 | 0.10 | c2 | c3 | c4 |
c(NO2) / mol �� L��1 | 0.00 | 0.12 | 0.20 | 0.22 | 0.22 | 0.22 |
���ݱ������ݺͱ����ṩ�������(c1��c2��c3��c4��ʾ��Ӧ��Ũ��)����ش����и�С�⣺
(1)�÷�Ӧ��ѧ����ʽ_________________________�� ����c2____c3(������������������������)
(2)c4��_______mol �� L��1����0��20 s��NO2��ƽ����Ӧ����Ϊ______________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2.80 g������þ��þ����ȫ����50.0 mL������Һ�μ�2.00 mol��L��1����������Һ��ǡ����ȫ����ʱ��ȥ200.0 mL�������õij������ա���ȴ������ù�������Ϊ4.40 g��
(1)��������������ʵ���Ũ��c(H2SO4)��____________________________��
(2)þ��������þ��þ�����ʵ���֮��Ϊn(MgO)�Un(Mg)��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��298Kʱ����n(HA)+n(A��)=0.lmol��HA��NaA���������ˮ�γ�1L��Һ����Һ��c(HA)��c(A��)��pH�Ĺ�ϵ��ͼ��ʾ��������������ȷ����
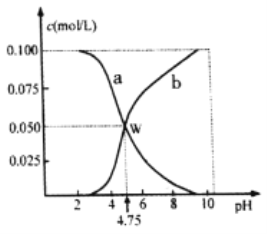
A. a�߱�ʾ����c(A��)�ı仯
B. pH=6 ʱ��c(Na+)+c(HA)>0. 1mol/L
C. 298Kʱ��A����ˮ��ƽ�ⳣ��(K)��������Ϊ10-10
D. ���������ʵ�����HA��NaA����ˮ�У�������ҺpHǡ��Ϊ4.75
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. Ǧ�����ڷŵ�����У�����������С��������������
B. SO3��Ba(NO3)2��Һ�ɵõ�BaSO4,SO2��Ba(NO3)2��Һ�ɵõ�BaSO3
C. �����£�SiO2(s)+3C(s)=SiC(s)+2CO(g)�����Է����У���÷�Ӧ�ġ�H<0
D. ��ӦA(g) ![]() B(g) ��H,������Ӧ�Ļ��ΪEakJ/mol���淴Ӧ�Ļ��ΪEbkJ/mol�����H=(Ea-Eb)kJ/mol
B(g) ��H,������Ӧ�Ļ��ΪEakJ/mol���淴Ӧ�Ļ��ΪEbkJ/mol�����H=(Ea-Eb)kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ���������;���ж�Ӧ��ϵ���ǣ� ��
A. SO2��ɱ����������������ʳƷ������
B. NH4HCO3�����ֽ⣬����������
C. ��������ˮ����������������������ԣ������ھ�ˮ
D. ����������Ũ�����Ӧ������������������Ũ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com