��2011?������ģ��ij��ѧ�С�������ͼ��ʾװ�ã����ּг�װ������ȥ��ʵ�飬��̽����ʪ��Cl
2��Na
2CO
3��Ӧ�IJ��

��1��д��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��
4HCl+MnO
2MnCl
2+Cl
2��+2H
2O
4HCl+MnO
2MnCl
2+Cl
2��+2H
2O
��
��2��д���Լ�Y�����ƣ�
����ʳ��ˮ�����Ȼ�����Һ��
����ʳ��ˮ�����Ȼ�����Һ��
��
��3����C�з�Ӧ��Ĺ������ʷֳ����ȷݷ�װ����֧�Թ��У���������һ֧�Թ��м�����ˮ��������ȫ�ܽ�μ�BaCl
2��Һ�������������ٵμ�NaOH��Һ����ǣ�д����������������ӷ���ʽ��
HCO3-+Ba2++OH-=BaCO3��+H2O
HCO3-+Ba2++OH-=BaCO3��+H2O
���ɴ������ƶϹ�������к���
NaHCO3
NaHCO3
���ѧʽ����ͬ����
������һ֧�Թ��еμӹ�����ϡ���ᣬ����ɫ��ζ�������������Һ���壬������Һ�μӹ�����AgNO
3��Һ����Һ����ǣ������ˡ�ϴ�ӡ�����õ�7.175g���壬�ɴ������ƶϹ�������к���
NaCl
NaCl
��
��4����֪C����0.1molCl
2�μӷ�Ӧ��D���ռ������������ȵ�һ���������ϣ�2�����������ݿ���֪��C�з�Ӧ�Ļ�ѧ����ʽΪ
2Cl2+2Na2CO3+H2O=2NaHCO3+2NaCl+Cl2O��
2Cl2+2Na2CO3+H2O=2NaHCO3+2NaCl+Cl2O��
��





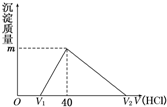 ��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������
��2011?������ģ����һ�����������Ͻ�����ˮ�У��Ͻ�ȫ���ܽ⣬�õ�20mL OH-Ũ��Ϊ1mol/L����Һ��Ȼ����1mol/L������ζ���������������������������ϵ��ͼ��ʾ��������ѡ����ȷ���ǣ�������