
��2L�ܱ������У��ϳɰ���ӦN2(g)��3H2(g)![]() 2NH3(g)�ﵽƽ�⡣��ش��������⣺
2NH3(g)�ﵽƽ�⡣��ش��������⣺
(1) ��֪��450��ʱNH3��Ũ�ȴ���550��ʱ��������Ӧ��______ __�ȷ�Ӧ��
(2) ��Ӧ�����У�0��2s��N2�����ʵ�����2mol��Ϊ1mol����ԣ�H2����___________��
(3) ��˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����_________ __��
A��c(N2)=c(H2)=c (NH3) B��������ѹǿ���ֲ���
C����(N2)=3��(H2) D�������ڵ��ܶȱ��ֲ���
(4) ��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ��Ĵ�ʩ��__________��
A����ʱ�����NH3���� B���ʵ������¶�
C. ����ѹǿ D.ѡ���Ч�Ĵ���
��1������(1��) (2��0.75mol/(L��s) (2��) (3��B (1��) (4��C(1��)
���⿼�黯ѧ��Ӧ���ʼ���ѧƽ���й�֪ʶ���ؼ�������ȷ��ѧ��Ӧ���ʵ�Ӱ�����ؼ���ѧƽ����жϡ�(1)450��ʱNH3��Ũ�ȴ���550��ʱ��˵���¶����ߣ�ƽ�����淴Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ��(2)�ԣ�H2��=3�ԣ�N2��=3(2-1)/(2��2)= 0.75mol/(L��s)��(3) A��Ũ����ȣ�����һ���ﵽƽ�⣬ֻ��Ũ�Ȳ��ڸı�ʱ����ʾ�ﵽ��ƽ�⣬�÷�Ӧ���������ȵķ�Ӧ������̶�����ѹǿ���䣬��ʾ�÷�Ӧ�Ѵ�ƽ�⣻C����δָ��������Ӧ���ʻ����淴Ӧ���ʣ��ʲ��ܱ�ʾ�÷�Ӧ�Ѵ�ƽ�⣻����̶����������䣬�ܶȲ��䣬�ʲ��ܱ�ʾ�÷�Ӧ�Ѵ�ƽ�⣻��ѡB��
(4����ʱ�����NH3���壬�����������С�淴Ӧ���ʣ�A�������⣻�ʵ������¶ȣ�ƽ�����淴Ӧ�����ƶ���B�������⣻����ѹǿ,��Ӧ��������ƽ��������Ӧ�����ƶ���C�����⣬��ȷ������ֻ�ı䷴Ӧ���ʣ���Ӱ��ƽ���ƶ���D�������⣬��ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| H | + 4 |
| O | - 2 |
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�
��Դ����Լ���ҷ�չ���̵�����֮һ���״��������ѵȱ���Ϊ2 1���͵���ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ�����и����ڶ��ε��п��Ի�ѧ�Ծ� ���ͣ������
��18�֣���1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ_________________________________������Ӧ������
ת����0��3mol���ӣ������������������____________g��
��2��ͬһ���ʳ���̬����ֵ���Һ̬����ֵ��֮����̬����ֵ��С����ͬ��ͬѹ��һ����ѧ��Ӧ�����������������ڷ�Ӧ�����������Ϳ��Դ�����Ϊ�÷�Ӧ���ر�Ϊ0��ij��ѧ��ȤС�飬ר���о�������Ԫ�ؼ���ijЩ������IJ������ʡ������������£�

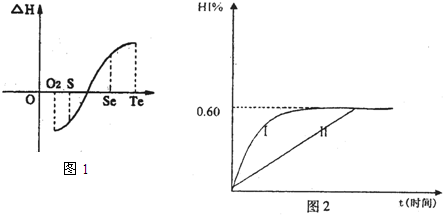
a���ڣ�Te��Ϊ���壬H2TeΪ���壬Te��H2����ֱ�ӻ�������H2Te
b�������ʵ�������������������H2��Ӧ���ʱ������ͼl��ʾ��
��ش��������⣺
H2���ϵķ�Ӧ______________��������ų��������ա�����������Ŀ������Ϣ�������ΪʲôTe��H2����ֱ�ӻ���_________________________________________.
��3���ڸ��ӵķ�Ӧ�У�Ҫ���Ƿ�Ӧ���Ⱥ�˳����֪ ��
�� ��2H2O
��2H2O Al��OH��3�� ��NH3��H2O�����е����ʵ�����
Al��OH��3�� ��NH3��H2O�����е����ʵ����� ��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
�ڶ������ӷ�Ӧ�����ӷ���ʽ��__________________________________.
������ӷ�Ӧ�����ӷ���ʽ��________________________________.
��4����1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��H2��g����I2��g�� 2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��

�ٴﵽƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ________mol��L.
�ڱ��ּ���ķ�Ӧ������ʵ������䣬���ı䷴Ӧ��������ijһ������HI���ı仯������l��ʾ��������������ǣ�д�����еĿ����ԣ�___________________________________________________________�����������£�ƽ�ⳣ��Kֵ____________�����������С���������䡱���ܱ��Ҳ���ܱ�С����
���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���1molH2��g����2molHI��g����������Ӧ�ﵽƽ��ʱ��H2���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��18�֣���1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ_________________________________������Ӧ������
ת����0��3mol���ӣ������������������____________g��
��2��ͬһ���ʳ���̬����ֵ���Һ̬����ֵ��֮����̬����ֵ��С����ͬ��ͬѹ��һ����ѧ��Ӧ�����������������ڷ�Ӧ�����������Ϳ��Դ�����Ϊ�÷�Ӧ���ر�Ϊ0��ij��ѧ��ȤС�飬ר���о�������Ԫ�ؼ���ijЩ������IJ������ʡ������������£�
a���ڣ�Te��Ϊ���壬H2TeΪ���壬Te��H2����ֱ�ӻ�������H2Te
b�������ʵ�������������������H2��Ӧ���ʱ������ͼl��ʾ��
��ش��������⣺
H2���ϵķ�Ӧ______________��������ų��������ա�����������Ŀ������Ϣ�������ΪʲôTe��H2����ֱ�ӻ���_________________________________________.
��3���ڸ��ӵķ�Ӧ�У�Ҫ���Ƿ�Ӧ���Ⱥ�˳����֪![]() ��
��![]() ��2H2O
��2H2OAl��OH��3�� ��NH3��H2O�����е����ʵ�����
![]() ��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
�ڶ������ӷ�Ӧ�����ӷ���ʽ��__________________________________.
������ӷ�Ӧ�����ӷ���ʽ��________________________________.
��4����1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��H2��g����I2��g��![]() 2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
�ٴﵽƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ________mol��L.
�ڱ��ּ���ķ�Ӧ������ʵ������䣬���ı䷴Ӧ��������ijһ������HI���ı仯������l��ʾ��������������ǣ�д�����еĿ����ԣ�___________________________________________________________�����������£�ƽ�ⳣ��Kֵ____________�����������С���������䡱���ܱ��Ҳ���ܱ�С����
���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���1molH2��g����2molHI��g����������Ӧ�ﵽƽ��ʱ��H2���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�����и����ڶ��ε��п��Ի�ѧ�Ծ� ���ͣ������
��18�֣���1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
д���÷�Ӧ�Ļ�ѧ����ʽ����ƽ_________________________________������Ӧ������
ת����0��3mol���ӣ������������������____________g��
��2��ͬһ���ʳ���̬����ֵ���Һ̬����ֵ��֮����̬����ֵ��С����ͬ��ͬ ѹ��һ����ѧ��Ӧ�����������������ڷ�Ӧ�����������Ϳ��Դ�����Ϊ�÷�Ӧ���ر�Ϊ0��ij��ѧ��ȤС�飬ר���о�������Ԫ�ؼ���ijЩ������IJ������ʡ������������£�
ѹ��һ����ѧ��Ӧ�����������������ڷ�Ӧ�����������Ϳ��Դ�����Ϊ�÷�Ӧ���ر�Ϊ0��ij��ѧ��ȤС�飬ר���о�������Ԫ�ؼ���ijЩ������IJ������ʡ������������£�
a���ڣ�Te��Ϊ���壬H2TeΪ���壬Te��H2����ֱ�ӻ�������H2Te
b�������ʵ�������������������H2��Ӧ���ʱ������ͼl��ʾ��
��ش��������⣺
H2���ϵķ�Ӧ______________��������ų��������ա�����������Ŀ������Ϣ�������ΪʲôTe��H2����ֱ�ӻ���_______________________________ _____
_____ _____.
_____.
��3���ڸ��ӵķ�Ӧ�У�Ҫ���Ƿ�Ӧ���Ⱥ�˳����֪ ��
��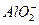 ��2H2O
��2H2O Al��OH��3�� ��NH3��H2O�����е����ʵ�����
Al��OH��3�� ��NH3��H2O�����е����ʵ����� ��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
��Al3����H�������Һ�У������μ�NaOH��Һ��ֱ�������������Ͻ��裬���η������������ӷ�Ӧ�����У�
�ڶ������ӷ�Ӧ�����ӷ���ʽ��__________________________________.
������ӷ�Ӧ�����ӷ���ʽ��________________________________.
��4����1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��H2��g����I2��g�� 2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��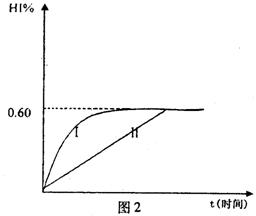
�ٴﵽƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ________mol��L.
�ڱ��ּ���ķ�Ӧ������ʵ������䣬���ı䷴Ӧ��������ijһ������HI���ı仯������l��ʾ��������������ǣ�д�����еĿ����ԣ�___________________________________________________________�����������£�ƽ�ⳣ��Kֵ____________�����������С���������䡱���ܱ��Ҳ���ܱ�С����
���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���1molH2��g����2molHI��g����������Ӧ�ﵽƽ��ʱ��H2���������Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com