

��1������װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ��________����д����������ʱ�����ῴ��________����дʵ��������֤����װ�õ����������á�
��2�����Թ��м���0.0005mol��L-1�ĵ�ˮ1.0mL��������������ˮϡ�ͺ��ټ���2��3���ε�����Һ�����Ƴ���ҺA���ⶨָ���ص�Ŀ�����SO2�ĺ���ʱ������ע�����Ļ���������
����A��Һ��________ɫ��Ϊ________ɫʱ��Ӧǡ����ȫ���У���ʱֹͣ�������÷�Ӧ�Ļ�ѧ����ʽΪ________��
��3���ҹ����������������ж�ÿ�ο��������ⶨ��SO2�����Ũ����ֵ���±���
Ũ����ֵ/mg��m-3
һ����
������
������
0.15
0.50
0.70
��ѧ������С��ֳɵ�һС��͵ڶ�С�飬ʹ����ͬ��ʵ��װ�ú���ҺA����ͬһ��
�㡢ͬʱ����������SO2�ĺ���������Ӧǡ����ȫ���У���¼�����������£�����ÿ�γ���500mL�����뽫�±���д����������ʱ����2λ��Ч���֣���
������������������������������������ ��һС�������������� �ڶ�С��
�������������������������������� 120���������������� 140
SO2����/mg��m-3��������������������������������������������������������������������
�жϸõص�Ŀ�����SO2�ĺ�������________�������֣�������________������һ���ڶ�����С��IJⶨ�����ȷ����һС��ʵ���������ϴ�ƫ���ԭ���ǣ�����С������װ�ú�ҩƷ�������⣩________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
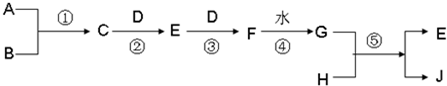
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

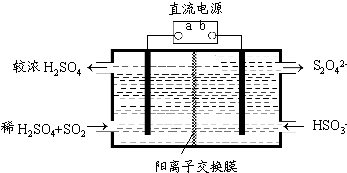
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ���������������ѧ�ڶ�ģ��ѧ�Ծ��������棩 ���ͣ������
�о�NO2 ��SO2 ��CO�ȴ�����Ⱦ��Ĵ���������Ҫ���塣NO2�������з�Ӧ��������
6 NO2(g)+8NH3(g) 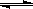 7N2(g)+12H2O(g)+Q��Q��0����
7N2(g)+12H2O(g)+Q��Q��0����
���������գ�
��1����Ӧ��ƽ�ⳣ������NO2��Ч�ʸߡ�����÷�Ӧƽ�ⳣ���Ĵ�ʩ�� ��
��2��һ��������������Ӧ��ij����̶����ܱ������н��У���˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
a��c(NO2)��c(NH3) = 3��4 b��6v(NO2) = 7v(N2)
c��������������ѹǿ���ٱ仯 d�������������ܶȲ��ٱ仯
��3����ƽ��ʱNO2��N2��Ũ��֮��Ϊm/n�����������������䣬��С��Ӧ�����������ﵽ�µ�ƽ�⣬��ʱNO2��N2��Ũ��֮�� m/n���>������=����<������
��4��ij�¶��£���һ���ݻ�Ϊ2���ķ�Ӧ�����ڣ�������Ӧ2���Ӻ�ﵽƽ�⣬��øպ���3mol���ӷ���ת�ƣ�����2������NH3��ƽ����Ӧ����Ϊ��
v(NH3) = ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com