
 2CrO42������ɫ����2H+
2CrO42������ɫ����2H+ 2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣�
2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������c (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����2�֣� 2CrO42������ɫ����2H+��֪�������A���ʿ�ʹƽ�������ƶ�������Ӧ���������ʣ��ų�ab������������л�ԭ�ԣ�����CrO42����Ӧ������Cr3+���������CrO42����Ӧ������ʹƽ�������ƶ�����ѡc��
2CrO42������ɫ����2H+��֪�������A���ʿ�ʹƽ�������ƶ�������Ӧ���������ʣ��ų�ab������������л�ԭ�ԣ�����CrO42����Ӧ������Cr3+���������CrO42����Ӧ������ʹƽ�������ƶ�����ѡc�� 2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������ʹc (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����pH���͡�
2CrO42������ɫ��+2H+������Ba(NO3)2��Һ����BaCrO4������ʹc (CrO42��)���ͣ�ƽ�������ƶ���c (H+)����pH���͡�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
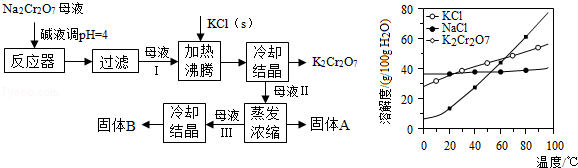
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
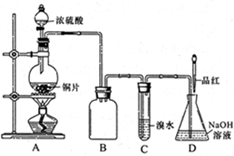
| ʵ����� | ʵ����� | ���� | ��Ӧ�Ŀ������ |
| �� | �μ��������۵⻯����Һ���� | | III |
| �� | �μ������غ�ɫ��KI3��Һ���� | | II |
| �� | �������������KMNO4��Һ���� | ��Һ����ɫ | |
| �� | ���뼸С��CaCO3���� | �����ݲ��� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
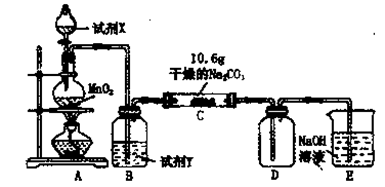
 ��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3����֪C����0��1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ�Ļ�ѧ����ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

| ���� | Al3�� | Fe3�� | Ni2�� |
| pH | 5.2 | 4.1 | 9.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
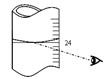
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 NH4NO2��NaCl
NH4NO2��NaCl NH3����HNO2
NH3����HNO2 N2O3����H2O
N2O3����H2O 2N2��3H2O
2N2��3H2O| N2��H2������� | 5��1 | 3��1 | 1��1 | 1��3 | 1��5 |
| ��̪���ɫ����ʱ��/min | 8��9 | 7��8 | 6��7 | 3��4 | 9��10 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| A����ѧ�� | B����ȫ�� | C�������� | D����Լ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com