
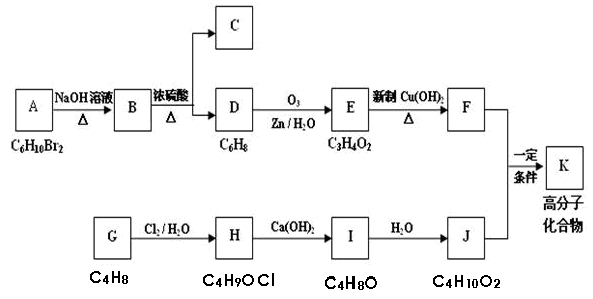
Θ®10Ζ÷Θ©Ρ≥”–ΜζΈοAΒΡΖ÷Ή” ΫΈΣC6H10Br2Θ§≤ΜΡή ΙδεΥ°Ά …ΪΘ§GΒΡΖ÷Ή” ΫΈΣC4H8Θ§Ρή ΙδεΥ°Ά …ΪΘ§AΓΔGΒΡΚΥ¥≈Ι≤’ώ«βΤΉ»γœ¬ΓΘGΈΣ ·”ΆΜ·ΙΛΜυ±Ψ‘≠ΝœΘ§KΈΣΗΏΖ÷Ή”Μ·ΚœΈοΘ§1mol J”κΉψΝΩΫπ τΡΤΖ¥”Π…ζ≥…22.4LΘ®±ξΉΦΉ¥ΩωΘ©«βΤχΘ§CΓΔDΜΞΈΣΆ§Ζ÷“λΙΙΧεΓΘΗς”–ΜζΈο÷°ΦδΒΡΉΣΜ·ΙΊœΒ»γœ¬ΘΚ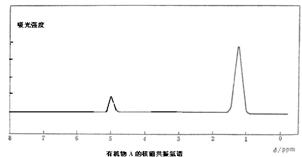
“―÷ΣΘΚ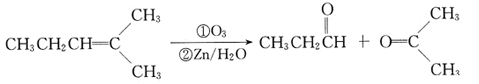
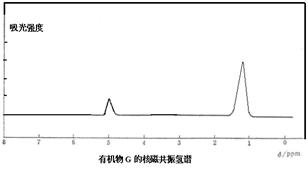
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©CΒΡΫαΙΙΦρ ΫΈΣ Θ§
Θ®2Θ©–¥≥ωBΓζDΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
Θ®3Θ©A‘Ύ“ΜΕ®ΧθΦΰœ¬“≤Ρή÷±Ϋ”Ζ¥”Π…ζ≥…DΘ§–¥≥ωœύ”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘ®–ηΉΔΟςΖ¥”ΠΧθΦΰΘ©
Θ®4Θ©–¥≥ωFΚΆJΖ¥”Π…ζ≥…KΒΡΜ·―ßΖΫ≥Χ Ϋ
Θ®5Θ©”–ΜζΈοR”κBΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§¬ζΉψœ¬Ν–ΧθΦΰΒΡRΙ≤”– ÷÷Θ§–¥≥ω»Έ“βΒΡΝΫ÷÷
ΔΌ ”κNaOH»ή“ΚΖ¥”Π ΔΎ≤ΜΡή”κNaHCO3»ή“ΚΖ¥”Π ΔέΧΦΝ¥Έό÷ßΝ¥
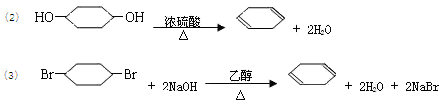
18ΓΔΘ®10Ζ÷Θ©(1)

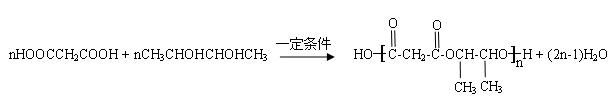
(4)
(5) 5 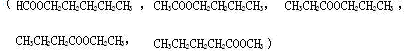
ΫβΈω ‘ΧβΖ÷ΈωΘΚA÷–ΫΪBrΜΜ≥…HΘ§ΦΤΥψ≤Μ±ΞΚΆΕ»ΈΣ1Θ§“ρ≤ΜΡή ΙδεΥ°Ά …ΪΘ§”ΠΗΟ «ΜΖΉ¥ΫαΙΙΓΘΚΥ¥≈Ι≤’ώ«βΤΉ”–ΝΫΗωΖεΘ§ΖεΟφΜΐ÷°±»ΈΣ1:4Θ§Ζ÷Ή” ΫΈΣC6H10Br2Θ§Ω…ΡήΈΣ1,4-ΕΰδεΜΖΦΚΆιΓΘ
NaOH»ή“ΚΘ§Φ”»»Θ§AΉΣΜ·ΈΣBΘΚ1,4-ΜΖΦΚΕΰ¥ΦΓΘ≈®ΝρΥαΆ―Υ°Ω…ΒΟΝΫ÷÷≤ζΈοΓΣΓΣDΘΚ1,4-ΜΖΦΚΕΰœ©ΚΆCΘΚ1,3-ΜΖΦΚΕΰœ©ΓΘD≥τ―θΜ·ΚσΒΟΒΫEΘΚ±ϊΕΰ»©ΓΘEΨ≠«β―θΜ·Ά≠–ϋΉ«“Κ―θΜ·ΒΟΒΫFΘΚ±ϊΕΰΥαΓΘGΡή»ΟδεΥ°Ά …ΪΘ§ΚΥ¥≈Ι≤’ώ«βΤΉ”–ΝΫΗωΖεΘ§ΖεΟφΜΐ÷°±»ΈΣ1:3Θ§Ζ÷Ή” ΫΈΣC4H8Θ§«“Ρή±Μ¥Έ¬»ΥαΦ”≥…Θ§Ω…÷ΣGΈΣ2-ΦΉΜυ±ϊœ©Μρ’Ώ2-ΕΓœ©Θ§ΫαΚœΚσΟφ–π ω”ΠΗΟ «2-ΕΓœ©ΓΘGΨ≠¥Έ¬»ΥαΦ”≥…ΒΟΒΫHΘΚ3-¬»-2-ΕΓ¥ΦΓΘHΜΖ―θΜ·ΒΟΒΫIΘΚ2,3-ΜΖ―θΕΓΆιΓΘI”κΥ°Φ”≥…ΒΟΒΫJΘΚ2,3-ΕΓΕΰ¥ΦΓΘ1mol J”κΉψΝΩΫπ τΡΤΖ¥”Π…ζ≥…22.4LH2Θ§22.4LΓ¬22.4L/mol=1molΘ§ΥΒΟς1molJΚ§”–2mol-OHΓΘ
ΩΦΒψΘΚ±ΨΧβ÷ς“ΣΩΦ≤λ―ß…ζ «Ζώ’ΤΈ’ΝΥΚΥ¥≈Ι≤’ώΘ§”–ΜζΜ·―ßΖ¥”Π÷°ΦδΒΡœύΜΞΉΣΜ·Θ§”–ΙΊΜ·―ßΖΫ≥Χ ΫΒΡΦΤΥψΓΘ
ΒψΤάΘΚ±ΨΧβΡ―Ε»¥σΘ§άϊ”ΟΚΥ¥≈Ι≤’ώ«βΤΉΆΦΆΤΥψ≥ωAΚΆGΒΡΫαΙΙ Ϋ «ΫβΧβΒΡΙΊΦϋΘ§ΉωΧβ ±“ΣœΗ–ΡΘ§“Μ≤Ϋ“Μ≤Ϋά¥ΓΘΤΫ ±ΉΔ“β÷Σ ΕΒψΒΡΜΐάέΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
 Ρ≥”–ΜζΈοAΒΡΖ÷Ή” ΫΈΣC5H10O2Θ§“―÷ΣAΓΪE”–»γΆΦΉΣΜ·ΙΊœΒΘ§«“D≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§CΓΔEΨυΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘ρAΒΡΫαΙΙΩ…Ρή”–Θ®ΓΓΓΓΘ©
Ρ≥”–ΜζΈοAΒΡΖ÷Ή” ΫΈΣC5H10O2Θ§“―÷ΣAΓΪE”–»γΆΦΉΣΜ·ΙΊœΒΘ§«“D≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§CΓΔEΨυΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘ρAΒΡΫαΙΙΩ…Ρή”–Θ®ΓΓΓΓΘ©≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
(1)AΒΡ Β―ι Ϋ «________________ΓΘ
(2)»τAΚΆNa2CO3»ή“ΚΖ¥”ΠΜλΚœ”–ΤχΧεΖ≈≥ωΘ§ΚΆ¥ΦΖΔ…ζθΞΜ·Ζ¥”ΠΘ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ____________ΓΘ
(3)»τA «“ΉΜ”ΖΔ”–Υ°ΙϊœψΈΕΒΡ“ΚΧεΘ§ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§‘ρΤδΫαΙΙΦρ ΫΈΣ_________ΓΘA‘ΎNaOH»ή“Κ÷–Υ°ΫβΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________________________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Θ®1Θ©ΗΟ”–ΜζΈοΒΡ Β―ι Ϋ «__________ΓΘ?
Θ®2Θ©»τA «“Μ÷÷Έό…ΪΨΏ”–«ΩΝ“¥ΧΦΛ–‘ΒΡΤχΧεΘ§ΨΏ”–ΜΙ‘≠–‘Θ§ΤδΫαΙΙΦρ Ϋ «__________ΓΘ
Θ®3Θ©»τAΚΆNa2CO3ΜλΚœ”–ΤχΧεΖ≈≥ωΘ§ΚΆ¥ΦΖΔ…ζθΞΜ·Ζ¥”ΠΘ§‘ρAΒΡΫαΙΙΦρ Ϋ «__________ΓΘ
Θ®4Θ©»τA «“ΉΜ”ΖΔΓΔ”–Υ°ΙϊœψΈΕΒΡ“ΚΧεΘ§ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§‘ρΤδΫαΙΙΦρ ΫΈΣ__________ΓΘ
Θ®5Θ©»τΤδΖ÷Ή”ΫαΙΙ÷–Κ§”–6ΗωΧΦ‘≠Ή”Θ§ΨΏ”–Εύ‘Σ¥ΦΚΆ»©ΜυΒΡ–‘÷ Θ§‘ρΤδΫαΙΙΦρ ΫΈΣ__________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Θ®1Θ©ΗΟ”–ΜζΈοΒΡ Β―ι ΫΈΣ ΓΘ?
Θ®2Θ©»τA «“Μ÷÷Έό…ΪΓΔΨΏ”–¥ΧΦΛ–‘ΤχΈΕΒΡΤχΧεΘ§«“”–ΜΙ‘≠–‘Θ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®3Θ©»τAΚΆNa2CO3ΜλΚœΘ§”–ΤχΧεΖ≈≥ωΘ§ΚΆ¥ΦΡήΖΔ…ζθΞΜ·Ζ¥”ΠΘ§‘ρAΒΡΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®4Θ©»τA «“ΉΜ”ΖΔΓΔ”–Υ°ΙϊœψΈΕΒΡ“ΚΧεΘ§ΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§‘ρΤδΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®5Θ©»τAΖ÷Ή”÷–Κ§”–6ΗωΧΦ‘≠Ή”Θ§ΨΏ”–Εύ‘Σ¥ΦΚΆ»©ΒΡ–‘÷ Θ§‘ρΤδΫαΙΙΦρ ΫΈΣΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com