
������AΪһ�ֳ�������Ԫ����һ�ֳ����ǽ���Ԫ����ɵĻ�����������������ӵĸ�����Ϊ2:3��KΪ������̬�ǽ������ʣ�J��NΪ������̬���ʣ�����Ϊ���������I��F�ڳ�����ΪҺ̬��C��DΪ�̼������壬H��ɫ��ζ���壬BΪ��ɫ��״����,LΪ�ȼҵ�еij�����Ʒ��F��Ũ��Һ��K���ȿ�����D��H��(����������δ���)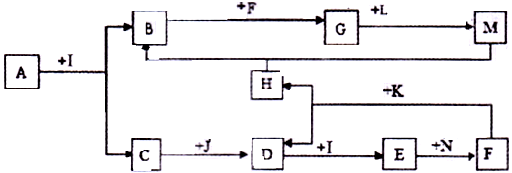
(1)H�ĽṹʽΪ: ��B�Ļ�ѧʽΪ: ��
(2)д�����б仯�Ļ�ѧ����ʽ:
A+I ��B+C�� ��
F��Ũ��Һ��K���ȿ�����D��H�� ��
(3)д�����б仯�����ӷ���ʽ:
Nͨ��E��ˮ��Һ: ��
M��ˮ��Һ��ͨ�˹�����H: ��
(4)��A��K��������������������ʵ���_ �����ڷǵ���ʵ��� (�ñ�Żش�)��
��14�֣���1��O��C��O ��1�֣���Al(OH)3��1�֣�
��2��Al2S3��6H2O��2Al(OH)3����3H2S����2�֣�
C��2H2SO4(Ũ) CO2����2SO2����2H2O��2�֣�
CO2����2SO2����2H2O��2�֣�
(3)H2SO3��Cl2��H2O��SO42����4H����2Cl����2�֣� AlO2����CO2��2H2O��Al(OH)3����HCO3����2�֣�
��4��B��C��E��I��2�֣���D��H��2�֣������һ���1�֣�ѡ��1�������÷֣�
�������������BΪ��ɫ��״��������BӦ��������������������AΪһ�ֳ�������Ԫ����һ�ֳ����ǽ���Ԫ����ɵĻ�����������������ӵĸ�����Ϊ2:3�����A��һ��������Ԫ�ء���Ԫ�ص���Ҫ���ϼ��ǣ�3�ۣ���A������һ��Ԫ�صĻ��ϼ�Ӧ���ǣ�2�ۣ���Ϊ�ڢ�A��Ԫ�ء�I��F�ڳ�����ΪҺ̬��������һ����ˮ����˵��AӦ����Al2S3��������Al2O3����ΪAl2O3������ˮ��Al2S3����ˮ��������������H2S����C��H2S��I��ˮ��J��NΪ������̬���ʣ�C�ܺ�J��Ӧ�����J��������DΪ�̼������壬��D��SO2��SO2����ˮ���������ᡣKΪ������̬�ǽ������ʣ�F��Ũ��Һ��K���ȿ�����D��H��H��ɫ��ζ���壬����HӦ����CO2��K��̼��F�����ᣬ���N���������������������ᷴӦ������������LΪ�ȼҵ�еij�����Ʒ��L�ܺ���������Ӧ������L���������ƣ�M��ƫ�����ơ�
��1���������Ϸ�����֪��H�ĽṹʽΪO��C��O��B�Ļ�ѧʽΪAl(OH)3��
��2���������Ϸ�����֪��A+I ��B+C �Ļ�ѧ����ʽΪAl2S3��6H2O��2Al(OH)3����3H2S����F��Ũ��Һ��K���ȿ�����D��H�Ļ�ѧ����ʽΪC��2H2SO4(Ũ) CO2����2SO2����2H2O��
CO2����2SO2����2H2O��
(3)�������Ϸ�����֪�� Nͨ��E��ˮ��Һ�����ӷ���ʽΪH2SO3��Cl2��H2O��SO42����4H����2Cl����M��ˮ��Һ��ͨ�˹�����H�����ӷ���ʽΪAlO2����CO2��2H2O��Al(OH)3����HCO3����
��4�����ڵ���ƽ��ĵ������������ʣ������A��K��������������������ʵ�������������H2S�������ᡢˮ������ѡB��C��E��I������ˮ��������״̬�²��ܵ���Ļ�����Ƿǵ���ʣ��������ڷǵ���ʵ���CO2��SO2����ѡD��H��
���㣺���������ƶϡ��ṹʽ������ʽ����д�Լ�������ʺͷǵ���ʵ��жϵ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��ѧ��������A��һ�������·������·�Ӧ��A+B��E+F+H2O��δ��ƽ��
��1����AΪС�մ�FΪ���塣�÷�Ӧ�����ӷ���ʽΪ ��
��2����AΪ�Ϻ�ɫ�������ʣ�����F��������λ��ͬһ����Ķ�����Ԫ����ɡ���Ӧ�Ļ�ѧ����ʽΪ_____________________��
��3����A�Ǵ��������Ҫ�ɷ֣�B�����ᡣд����Ӧ�Ļ�ѧ����ʽΪ ��
��4����AΪ����ɫ���嵥�ʣ�F�ļ�����Һ���շ�����SO2�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ����ѧ��ѧijЩ����֮����һ�������µ��ת����ϵ����֪A��һ�ֳ�����Һ̬������,C��D��G��H��K�ǵ��ʣ�����Ϊ�����G��K����ͨ���е�������ҪԪ�أ�����K�����٣�E��F��Ӧ��Һ��������෴��F����ɫ��ӦΪ��ɫ���밴Ҫ������
��1��д��������ĵ���ʽ��_________________________��
��2����д��A��J��Ӧ�����ӷ���ʽ��_______________________________________��
�ڽ�������Hͨ��F����Һ�У���Ӧ�������ӷ���ʽΪ��_________________________��
��3��B������ϡ���ᷴӦ�����μӷ�Ӧ������Ϊ4mol��ת�Ƶ��ӵ����ʵ���Ϊ_________mol������2λ��Ч���֣���
��4����֪��200�棬101Kpa�£�0��12g����K��A��ȫ��Ӧ����C��I��������1316J��������д���˷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
��5������PtΪ�缫����I��D�Լ�F����Һ���ԭ��أ��������ĵ缫��ӦΪ��___________��
�����ô˵�ص��300mL5mol��L���Ȼ�����Һһ��ʱ�䣬�������ռ�����״����3��36L���壬��ʱ��Һ��pHΪ_________��������ǰ����Һ������仯����
�������õ���������Һ��ͨ�������̼����4��48L����״��������ʱ��Һ����������Ũ�ȵĹ�ϵ�ɴ�С��˳��Ϊ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� �� ��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ����
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ���� ��4����Ӧ�۲�����K�Ļ�ѧʽΪ ��
��4����Ӧ�۲�����K�Ļ�ѧʽΪ �� ��5����Ӧ�ܵ����ӷ���ʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A�������������������Ľ�����BΪ����ɫ��ĩ���ڳ�����CΪ��ɫҺ�壬E��G��H��I��JΪ���壬����HΪ����ɫ������Ϊ��ɫ��J��ʹʪ��ĺ�ɫʯ����ֽ������ͼ�в��ֲ�������ȥ����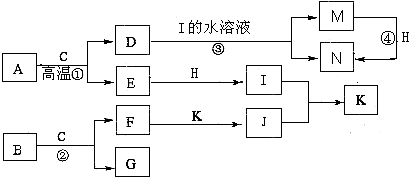
����д���пհ�
��1��д����ѧʽ��D ��K ��
��2��д���ڵ����ӷ���ʽ ��
��3����Ӧ�ܲ��������к��ַ�Ӧ���� ������ĸ��ţ���
A�����Ϸ�Ӧ B��������ԭ��Ӧ C�����ӷ�Ӧ D���û���Ӧ
��4����Ҫȷ���۷�Ӧ��������Һ�к���M���ʣ���ѡ�Լ�Ϊ ������ţ���
A��KSCN��Һ����ˮ B�����ۺ�KSCN
C��Ũ��ˮ D�����Ը��������Һ
��5����F���뵽M����Һ�ﲢ¶���ڿ����У����Թ۲쵽�������̵������ǣ�
��
��6�������£�H�������J��Ӧ����Ũ��İ��̣���һ�������ǿ�������Ҫ�ɷ�֮һ����д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ����һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʺͷ�Ӧ��������ȥ�����³�ѹ�£�������A�Ǽ�ͥ�����г��õ�һ�ֵ�ζƷ��A��C����ɫ��Ӧ���ʻ�ɫ��8����ɫ��ζ��Һ�壬D�ǻ���ɫ���壬E��F������ɫ���壬F��ˮ��Һ�����ᣬH��ˮ��Һ����Ư�ס�ɱ���ԣ�J��ˮ��Һ������ʱΪ���ɫ������
(1)H�Ļ�ѧʽΪ ��
(2)F�ĵ���ʽΪ ��
(3)��Ӧ�ٵĻ�ѧ����ʽΪ ��
(4)��Ӧ�ڵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵������ȷ���ǣ� ��
��Al2O3�����ͻ���ϡ�Al(OH)3������ȼ��
������ڹ����Ĵ�����ȼ�տ�������SO3
������SO2ͨ��Ũ��CaCl2��Һ�����ɰ�ɫ����
�ܹ�ҵ������ˮ��Ͳ������õ���ԭ����ʯ��ʯ
���ڹ�ҵ������������Ũ���ᷴӦ��ȡ������
��ҵ�ϳ��ù�������ά
��þ��ұ���� 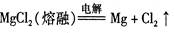
�����ȵĴ�����Һ��ϴ���ۣ�
����������ʴ������4HF+SiO2=SiF4��+2H2O
����Ȼ���в���������̬�Ĺ裬����Ҫ�Զ�����������ε���ʽ����
| A���ڢۢݢޢ� | B���٢ۢޢ� | C���ܢݢ�� | D���ڢۢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������͢����ȷ�����������ϵ����
| ѡ�� | ����I | ����II |
| A | NH4ClΪǿ�������� | ���ȿɳ�ȥNaCl�е�NH4Cl |
| B | Fe3+���������� | ��KSCN��Һ���Լ���Fe3+ |
| C | �ܽ�ȣ�CaCO3<Ca(HCO3)2 | �ܽ�ȣ�Na2CO3<NaHCO3 |
| D | SiO2����HF��Ӧ | �����ܱ����ڲ���ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵������ȷ����
| A����ͭ�к��е���Ҫ�Ͻ�Ԫ��������Ǧ |
| B��װ��Ũ�������������ж��������ˮ��ϴ�����ڲ� |
| C���뵼�幤ҵ��˵�ġ���ɳ̲���û�����ָ�����������Ƴɾ���� |
| D�����Ĺ̶�ֻ���ڸ��¡���ѹ�������������²���ʵ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com