
ŹµŃéŹŅÓĆÅØĮņĖįÓėĶµÄ·“Ó¦ÖĘȔɣĮæNaHSO3£¬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£ŗ
£Ø1£©×°ÖĆŅŅµÄ×÷ÓĆŹĒ_____________________”£
£Ø2£©×°Öƶ”µÄ×÷ÓĆŹĒĪüŹÕĪŪČ¾æÕĘųµÄSO2ĘųĢ壬Ęä·“Ó¦µÄĄė×Ó·½³ĢĪŖ ”£
£Ø3£© SO2 ĘųĢåÓŠĘư׊Ō”¢»¹ŌŠŌŗĶŃõ»ÆŠŌ”£½«SO2 ĶØČėĀČĖ®ÖŠ£¬SO2±ķĻֵďĒ________ŠŌ£¬»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø4£©·“Ó¦¹ż³ĢÖŠ£¬½«±ūÖŠµĪ¹ÜĄļµÄĘ·ŗģČÜŅŗµĪČė׶ŠĪĘ棬ČōĻÖĻóĪŖ £¬ŌņČÜŅŗÖŠµÄNaOHĶźČ«×Ŗ»ÆĪŖĮĖNaHSO3”£
£Ø5£©Čō±ū֊ƻӊ¼ÓČėĘ·ŗģČÜŅŗ£¬Ōņ²»ÄÜ×¼Č·ÅŠ¶ĻĒāŃõ»ÆÄĘŹĒ·ńĶźČ«×Ŗ»Æ”£ĻÖÓŠæɹ©Ń”ŌńµÄŅĒĘ÷ŗĶŹŌ¼Į£ŗÉÕ±”¢ŹŌ¹Ü”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü£» 2 mol/LŃĪĖį”¢2 mol/LĻõĖį”¢1 mol/LĀČ»Æ±µČÜŅŗ”¢l mol/LĒāŃõ»Æ±µČÜŅŗ”¢Ę·ŗģČÜŅŗ”¢ÕōĮóĖ®”£
ĒėÉč¼ĘŹµŃéĢ½¾æĪüŹÕŗó²śĪļÖŠŹĒ·ń“ęŌŚNaHSO3 ŗĶNa2SO3£¬½«ŹµŃé²Ł×÷”¢Ō¤ĘŚµÄŹµŃéĻÖĻóŗĶ½įĀŪĢīŌŚĻĀ±ķÖŠ”£
| ŹµŃé²Ł×÷ | Ō¤ĘŚĻÖĻóÓė½įĀŪ |
| ²½Öč1£ŗȔɣĮæ“ż²āŅŗ·ÅČėŹŌ¹ÜÖŠ£¬µĪ¼Ó¹żĮælmol/LĀČ»Æ±µČÜŅŗ”£¾²ÖĆŅ»¶ĪŹ±¼äŗó£¬µĆµ½ĀĖŅŗAŗĶ¹ĢĢåB”£ | |
| ²½Öč2£ŗĶł¹ĢĢåBÖŠ¼ÓČėÕōĮóĖ®Ļ“µÓ³Įµķ£¬¾²ÖĆŗóʜȄÉĻ²ćĒåŅŗ£¬Ļņ¹ĢĢåµĪČė2µĪ£Ø»ņÉŁĮæ£©Ę·ŗģ£¬ŌŁ | ČōĘ·ŗģĶŹÉ«£Ø»ņÓŠĘųÅŻ£©£¬Ōņ |
| ²½Öč3£ŗ | Čō Ōņ £» ·ńŌņ ”£ |
£Ø17·Ö£©
£Ø1£©£Ø2·Ö£©·ĄÖ¹±ūÖŠŅŗĢåµ¹ĪüČė×°ÖĆ¼×ÖŠ£Ø»ņ»ŗ³åĘæµÄ×÷ÓĆ£©””””
£Ø2£©£Ø3·Ö£© 5SO2£«2MnO4££«2H2O=5SO42££«2Mn2+£«4H+
£Ø3£©3·Ö£©»¹ŌŠŌ£Ø1·Ö£© SO2£«Cl2£«2H2O=H2SO4£«2HCl£Ø2·Ö£©
£Ø4£©£Ø2·Ö£©Ę·ŗģČÜŅŗĶŹÉ«
£Ø5£©£Ø7·Ö£©ŹµŃé²Ł×÷ Ō¤ĘŚĻÖĻóÓė½įĀŪ ²½Öč2£ŗŌŁµĪČė¹żĮæ2mol/LŃĪĖį£Ø1·Ö£©£¬Õńµ“£Ø1·Ö£© ²śĪļÖŠ“ęŌŚNa2SO3£Ø1·Ö£© ²½Öč3£ŗÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£Ø1·Ö£©£¬ĻņĘäÖŠ
¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį”££©£¬Õńµ“£Ø1·Ö£©Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ²śĪļÖŠ“ęŌŚNaHSO3£Ø1·Ö£©£»·ńŌņ²»“ęŌŚNaHSO3£Ø1·Ö£©”£
ĘĄ·ÖĖµĆ÷£ŗ
£Ø1£©ĪÄ×ÖÖŠÖ»ŅŖŠ“³ö¼ÓÓŠ×ÅÖŲŗŵÄĪÄ×Ö”¢»ÆѧŹ½»ņĄąĖʵÄŅāĖ¼£¬øĆĘĄ·Öµćøų·Ö£»
£Ø2£©ÓĆĘ·ŗģ¼ģŃéSO32£»ņHSO3£Ź±£ŗ¢ŁČē¹ūĻČ¼ÓŃĪĖį”¢ŗó¼ÓĘ·ŗģ£¬SO2ĮæÉŁĒŅ»Ó·¢Ņ»²æ·Ö£¬æÉÄÜ»į³öĻÖĘ·ŗģ²»ĶŹÉ«¶ųĪóÅŠ£¬ÕāÖÖĒéæöĮ½“¦ŗĻĘšĄ“æŪ1·Ö£»¢ŚČē¹ū²»¼ÓĘ·ŗģ”¢Ö»µĪŃĪĖį£¬Į½“¦ŗĻĘšĄ“æŪ1·Ö”£
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¶ĮĶ¼æÉÖŖ£¬×°ÖĆ¼×ÖŠCu+2H2SO4(ÅØ)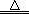 CuSO4+SO2”ü+2H2O£¬SO2²»½öŅ×ČÜÓŚĖ®£¬¶ųĒŅŅ×ČÜӌװÖƱūÖŠµÄNaOHČÜŅŗ£¬Ņņ“Ė±ūÖŠČÜŅŗŅ×µ¹Īü½ųČė¼××°ÖĆÄŚµÄÅØĮņĖįÖŠ£¬Ņ×Ōģ³É°²Č«ŹĀ¹Ź£¬ĖłŅŌ×°ÖĆŅŅµÄ×÷ÓĆ¾ĶŹĒ·ĄÖ¹±ūÖŠŅŗĢåµ¹ĪüČė×°ÖĆ¼×ÖŠ£Ø»ņĘš»ŗ³åĘæ»ņ°²Č«ĘæµÄ×÷ÓĆ£©£»£Ø2£©¶”×°ÖĆÖŠKMnO4×÷ĒæŃõ»Æ¼Į£¬SO2×÷»¹Ō¼Į£¬ĆĢŌŖĖŲÓÉ+7¼Ū½µĪŖ+2¼Ū£¬ĮņŌŖĖŲÓÉ+4¼ŪÉżĪŖ+6¼Ū£¬øł¾Ż»ÆŗĻ¼ŪÉż½µ×ÜŹżĻąµČ”¢Ō×ÓøöŹżŹŲŗćæÉÖŖ£¬2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4£¬»ņÕß5SO2£«2MnO4££«2H2O=5SO42££«2Mn2+£«4H+£»£Ø3£©ĀČĖ®ŹĒĒæŃõ»Æ¼Į£¬æÉŅŌ½«SO2Ńõ»ÆĪŖSO42££¬¼“SO2+Cl2+2H2O=H2SO4+2HCl£¬ŌņSO2ŹĒ»¹Ō¼Į£¬ĻŌ»¹ŌŠŌ£»£Ø4£©ČōSO2²»×ć£¬±ūÖŠ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£»ČōSO2¹żĮ棬±ūÖŠĻČ·¢ÉśµÄ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£¬ŗó·¢ÉśµÄ·“Ó¦ĪŖNa2SO3+H2O+SO2=2NaHSO3£¬ÓÉ“ĖĶʶĻ±ūÖŠSO2¹żĮ棬ŌņµĪČėĘ·ŗģČÜŅŗ»įĶŹÉ«£¬ŅņĪŖ¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō£»£Ø5£©Na2SO3ÓėBaCl2Ņ×·“Ӧɜ³ÉBaSO3³Įµķ£¬¶ųNaHSO3ÓėBaCl2²»ÄÜ·“Ó¦£»BaSO3Ņ×ČÜÓŚŃĪĖį£¬²¢·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬Ņņ“Ė²½Öč2ÖŠĻČĻņĻ“µÓŗóµÄ¹ĢĢåµĪČė2µĪ»ņÉŁĮæĘ·ŗģČÜŅŗ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£¬Õńµ“£¬Čō¹ĢĢåĶźČ«Čܽā£¬Ę·ŗģĶŹÉ«»ņÓŠĘųÅŻ£¬Ōņ×°ÖƱūµÄ²śĪļÖŠŗ¬ÓŠNa2SO3£»ÓÉÓŚæÉČÜŠŌµÄNaHSO3Ņ×ÓėBa(OH)2·“Ӧɜ³ÉBaSO3³Įµķ£¬»ņÕßNaHSO3Ņ×ÓėŃĪĖį·“Ó¦·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬¹Ź²½Öč3ÖŠæÉÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£¬ĻņĘäÖŠ¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£©£¬Õńµ“£¬Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ×°ÖƱūµÄ²śĪļÖŠ“ęŌŚNaHSO3£»·ńŌņ²»“ęŌŚNaHSO3”£
CuSO4+SO2”ü+2H2O£¬SO2²»½öŅ×ČÜÓŚĖ®£¬¶ųĒŅŅ×ČÜӌװÖƱūÖŠµÄNaOHČÜŅŗ£¬Ņņ“Ė±ūÖŠČÜŅŗŅ×µ¹Īü½ųČė¼××°ÖĆÄŚµÄÅØĮņĖįÖŠ£¬Ņ×Ōģ³É°²Č«ŹĀ¹Ź£¬ĖłŅŌ×°ÖĆŅŅµÄ×÷ÓĆ¾ĶŹĒ·ĄÖ¹±ūÖŠŅŗĢåµ¹ĪüČė×°ÖĆ¼×ÖŠ£Ø»ņĘš»ŗ³åĘæ»ņ°²Č«ĘæµÄ×÷ÓĆ£©£»£Ø2£©¶”×°ÖĆÖŠKMnO4×÷ĒæŃõ»Æ¼Į£¬SO2×÷»¹Ō¼Į£¬ĆĢŌŖĖŲÓÉ+7¼Ū½µĪŖ+2¼Ū£¬ĮņŌŖĖŲÓÉ+4¼ŪÉżĪŖ+6¼Ū£¬øł¾Ż»ÆŗĻ¼ŪÉż½µ×ÜŹżĻąµČ”¢Ō×ÓøöŹżŹŲŗćæÉÖŖ£¬2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4£¬»ņÕß5SO2£«2MnO4££«2H2O=5SO42££«2Mn2+£«4H+£»£Ø3£©ĀČĖ®ŹĒĒæŃõ»Æ¼Į£¬æÉŅŌ½«SO2Ńõ»ÆĪŖSO42££¬¼“SO2+Cl2+2H2O=H2SO4+2HCl£¬ŌņSO2ŹĒ»¹Ō¼Į£¬ĻŌ»¹ŌŠŌ£»£Ø4£©ČōSO2²»×ć£¬±ūÖŠ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£»ČōSO2¹żĮ棬±ūÖŠĻČ·¢ÉśµÄ·“Ó¦ĪŖSO2+2NaOH=Na2SO3+H2O£¬ŗó·¢ÉśµÄ·“Ó¦ĪŖNa2SO3+H2O+SO2=2NaHSO3£¬ÓÉ“ĖĶʶĻ±ūÖŠSO2¹żĮ棬ŌņµĪČėĘ·ŗģČÜŅŗ»įĶŹÉ«£¬ŅņĪŖ¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō£»£Ø5£©Na2SO3ÓėBaCl2Ņ×·“Ӧɜ³ÉBaSO3³Įµķ£¬¶ųNaHSO3ÓėBaCl2²»ÄÜ·“Ó¦£»BaSO3Ņ×ČÜÓŚŃĪĖį£¬²¢·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬Ņņ“Ė²½Öč2ÖŠĻČĻņĻ“µÓŗóµÄ¹ĢĢåµĪČė2µĪ»ņÉŁĮæĘ·ŗģČÜŅŗ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£¬Õńµ“£¬Čō¹ĢĢåĶźČ«Čܽā£¬Ę·ŗģĶŹÉ«»ņÓŠĘųÅŻ£¬Ōņ×°ÖƱūµÄ²śĪļÖŠŗ¬ÓŠNa2SO3£»ÓÉÓŚæÉČÜŠŌµÄNaHSO3Ņ×ÓėBa(OH)2·“Ӧɜ³ÉBaSO3³Įµķ£¬»ņÕßNaHSO3Ņ×ÓėŃĪĖį·“Ó¦·Å³öÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄSO2ĘųĢ壬¹Ź²½Öč3ÖŠæÉÓĆŹŌ¹ÜČ”ŹŹĮæĀĖŅŗA£¬ĻņĘäÖŠ¼ÓČė¹żĮælmol/LĒāŃõ»Æ±µČÜŅŗ£Ø»ņµĪČė2µĪĘ·ŗģ£¬ŌŁµĪČė¹żĮæ2mol/LŃĪĖį£©£¬Õńµ“£¬Čō³öĻÖ»ė×Ē£Ø»ņŗģÉ«ĶŹČ„£©£¬Ōņ×°ÖƱūµÄ²śĪļÖŠ“ęŌŚNaHSO3£»·ńŌņ²»“ęŌŚNaHSO3”£
æ¼µć£ŗæ¼²éĢ½¾æŹµŃé·½°øµÄÉč¼Ę£¬Éę¼°ĘųĢåÖʱø”¢ŠŌÖŹ”¢Ī²Ęų“¦ĄķµČ»ÆѧŹµŃ锣


 ¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£®[ŹµŃé»Æѧ]
±½ŃĒ¼×»ł±½ŅŅĶŖĖ×³Ę²é¶ūĶŖ£¬µ»ĘÉ«Ąāד¾§Ģ壬ČŪµć58”ę£¬·Šµć208”ę£Ø3.3kPa£©Ņ×ČÜÓŚĆŃ”¢ĀČ·ĀŗĶ±½£¬Ī¢ČÜÓŚ“¼”£ÖʱøŌĄķČēĻĀ£ŗ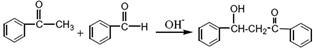
 (²é¶ūĶŖ)
(²é¶ūĶŖ)
£Ø1£©Öʱø¹ż³ĢÖŠ£¬ŠčŌŚ½Į°čĻĀµĪ¼Ó±½¼×Č©£¬²¢æŲÖʵĪ¼ÓĖŁ¶ČŹ¹·“Ó¦ĪĀ¶ČĪ¬³ÖŌŚ25”«30”ę£¬ĖµĆ÷øĆ·“Ó¦ŹĒ £ØĢī·ÅČČ»ņĪüČČ£©·“Ó¦”£ČēĪĀ¶Č¹żøߏ±£¬ŌņæÉŅŌ²ÉČ” “ėŹ©”£
£Ø2£©²śĘ·½į¾§Ē°£¬æÉŅŌ¼ÓČė¼øĮ£³ÉĘ·µÄ²é¶ūĶŖ£¬Ęä×÷ÓĆŹĒ ”£
£Ø3£©½į¾§ĶźČ«ŗ󣬊č³éĀĖŹÕ¼Æ²śĪļ”£³éĀĖ×°ÖĆĖł°üŗ¬µÄŅĒĘ÷³ż
¼õŃ¹ĻµĶ³Ķā»¹ÓŠ ”¢ (ĢīŅĒĘ÷Ćū³Ę)”£
£Ø4£©»ńµĆµÄÉīÉ«“Ö²śĘ·¼ÓČė»īŠŌĢ棬ŅŌ95%ŅŅ“¼ÖŲ½į¾§”£¼ÓČė»īŠŌĢæµÄ×÷ÓĆŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¹ĢĢ¬»ÆŗĻĪļAÓÉĮ½ÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³É£¬æÉÓėĖ®·¢Éśø“·Ö½ā·“Ó¦£¬¼××éĶ¬Ń§ÓĆĻĀĶ¼×°ÖĆ(¼Š³Ö×°ÖĆĀŌ)¶ŌĘä½ųŠŠĢ½¾æŹµŃ锣
(1)ŅĒĘ÷BµÄĆū³ĘŹĒ________”£
(2)ŹµŃéÖŠ£¬¢ņÖŠµÄŹŌÖ½±äĄ¶£¬¢ōÖŠŗŚÉ«·ŪÄ©Öš½„±äĪŖŗģÉ«²¢ÓŠMÉś³É£¬Ōņ¢óÖŠµÄŹŌ¼ĮĪŖ________£»¢ōÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________£»VÖŠµÄŹŌ¼ĮĪŖ________”£
(3)ŅŅ×éĶ¬Ń§½ųŠŠĶ¬ŃłŹµŃ飬µ«×°ÖĆĮ¬½ÓĖ³ŠņĪŖ¢ń¢ó¢ō¢ņV¢ö£¬“ĖŹ±¢ņÖŠĻÖĻóĪŖ________£¬ŌŅņŹĒ________”£
(4)¾ÉĻŹö·“Ó¦£¬2.5 g»ÆŗĻĪļAĄķĀŪÉĻæÉÖʵĆ0.56 L(±ź×¼×“æö)M£¬ŌņAµÄ»ÆѧŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖŃŠ¾æĶÓėÅØĮņĖįµÄ·“Ó¦£¬Ä³»ÆѧŠĖȤŠ”×é½ųŠŠČēĻĀŹµŃ锣
ŹµŃéI·“Ó¦²śĪļµÄ¶ØŠŌĢ½¾æ
°“ĻĀĶ¼×°ÖĆ£Ø¹Ģ¶Ø×°ÖĆŅŃĀŌČ„£©½ųŠŠŹµŃé
£Ø1£©AÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
£Ø2£©FÉÕ±ÖŠµÄČÜŅŗĶس£ŹĒ”””””””””””””””””””””””””””””””””£
£Ø3£©ŹµŃé¹ż³ĢÖŠ£¬ÄÜÖ¤Ć÷ÅØĮņĖįÖŠĮņŌŖĖŲµÄŃõ»ÆŠŌĒæÓŚĒāŌŖĖŲµÄĻÖĻóŹĒ””””””””””””
£Ø4£©ŹµŃé½įŹųŗó£¬Ö¤Ć÷A×°ÖĆŹŌ¹ÜÖŠ·“Ó¦ĖłµĆ²śĪļŹĒ·ńŗ¬ÓŠĶĄė×ӵIJŁ×÷·½·ØŹĒ”””” ”£
£Ø5£©ĪŖĖµĆ÷ÅØĮņĖįÖŠµÄĖ®ŹĒ·ńÓ°ĻģB×°ÖĆĻÖĻóµÄÅŠ¶Ļ£¬»¹Šė½ųŠŠŅ»“ĪŹµŃ锣ŹµŃé·½°øĪŖ ”””””””””””””””””””””””””””””””””””””””””””””””””””””£
ŹµŃé¢ņ””·“Ó¦²śĪļµÄ¶ØĮæĢ½¾æ
£Ø6£©ŌŚĶÓėÅØĮņĖį·“Ó¦µÄ¹ż³ĢÖŠ£¬·¢ĻÖÓŠŗŚÉ«ĪļÖŹ³öĻÖ£¬¾²éŌÄĪÄĻ×»ńµĆĻĀĮŠ×ŹĮĻ”£
׏ĮĻ1£ŗ
׏ĮĻ2:XÉäĻß¾§Ģå·ÖĪö±ķĆ÷£¬ĶÓėÅØĮņĖį·“Ӧɜ³ÉµÄŗŚÉ«ĪļÖŹĪŖCu2S”¢CuS”¢Cu7S4ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ”£
½öÓÉÉĻŹö׏ĮĻæÉµĆ³öµÄÕżČ·½įĀŪŹĒ”””””””””” ”£
a£®ĶÓėÅØĮņĖį·“Ó¦Ź±ĖłÉę¼°µÄ·“Ó¦æÉÄܲ»Ö¹Ņ»øö
b£®ĮņĖįÅضČŃ”ŌńŹŹµ±£¬æɱÜĆā×īŗó²śĪļÖŠ³öĻÖŗŚÉ«ĪļÖŹ
c£®øĆ·“Ó¦·¢ÉśµÄĢõ¼žÖ®Ņ»ŹĒĮņĖįÅØ¶Č”Ż15 mol”¤L-1
d£®ĮņĖįÅضČŌ½“ó£¬ŗŚÉ«ĪļÖŹŌ½æģ³öĻÖ”¢Ō½ÄŃĻūŹ§
£Ø7£©ĪŖ²ā³öĮņĖįĶµÄ²śĀŹ£¬½«øĆ·“Ó¦ĖłµĆČÜŅŗÖŠŗĶŗóÅäÖĘ³É250.00 mLČÜŅŗ£¬Č”øĆČÜŅŗ25.00 mL¼ÓČė×ćĮæKIČÜŅŗÕńµ“£¬ŅŌµķ·ŪČÜŅŗĪŖÖøŹ¾¼Į£¬ÓĆb mol”¤L-1Na2S2O3ČÜŅŗµĪ¶ØÉś³ÉµÄI2£¬3“ĪŹµŃéĘ½¾łĻūŗÄøĆNa2S2O3ČÜŅŗV mL”£Čō·“Ó¦ĻūŗÄĶµÄÖŹĮæĪŖag£¬ŌņĮņĖįĶµÄ²śĀŹĪŖ”””””””””””””””””” ”£”” £ØŅŃÖŖ£ŗ2Cu2++4I££½2CuI+I2£¬2S2O32£+I2£½S4O62£+2I££©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖĢ½Ė÷¹¤Ņµ·ĻĮĻµÄŌŁĄūÓĆ£¬Ä³»ÆѧŠĖȤŠ”×éÉč¼ĘĮĖČēĻĀŹµŃéĮ÷³Ģ£¬ÓĆŗ¬ÓŠĀĮ”¢ĢśŗĶĶµÄŗĻ½š·ĻĮĻÖĘČ”ĀČ»ÆĀĮ”¢ĀĢ·Æ¾§Ģå(FeSO4”¤7H2O)ŗĶµØ·Æ¾§Ģ唣
Ēė»Ų“š£ŗ
£Ø1£©Š“³ö²½Öč¢ń·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø2£©ŹŌ¼ĮXŹĒ ”£²½Öč¢ń”¢¢ņ”¢¢óÖŠ¾łŠč½ųŠŠµÄŹµŃé²Ł×÷ŹĒ ”£
£Ø3£©½ųŠŠ²½Öč¢ņŹ±£¬øĆŠ”×éÓĆČēĶ¼ĖłŹ¾×°ÖĆ¼°ŹŌ¼ĮÖĘČ”CO2²¢½«ÖʵƵÄĘųĢåĶØČėČÜŅŗAÖŠ”£Ņ»¶ĪŹ±¼äŗ󣬹Ū²ģµ½ÉÕ±ÖŠ²śÉśµÄ°×É«³Įµķ»įÖš½„¼õÉŁ”£ĪŖĮĖ±ÜĆā¹ĢĢåC¼õÉŁ£¬æɲÉČ”µÄøĽų“ėŹ©ŹĒ ”£
£Ø4£©ÓĆ¹ĢĢåFÖʱøCuSO4ČÜŅŗ£¬æÉÉč¼ĘŅŌĻĀČżÖÖĶ¾¾¶£ŗ
Š“³öĶ¾¾¶¢ŁÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ £¬ĒėŃ”³öÄćČĻĪŖµÄ×ī¼ŃĶ¾¾¶²¢ĖµĆ÷Ń”ŌńµÄĄķÓÉ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijĶ¬Ń§ĀĆÓĪŹ±·¢ĻÖ£¬Ćē×åČĖµÄŅųŹĪĆĄĄö¶ųø»ÓŠĆń×åĪĻƣ¬ÖĘ×÷ŅųŹĪŹ±æÉŅŌŃ”ÓĆFe£ØNO3)3ČÜŅŗ×öŹ“æĢ¼Į”£ŹÜ“ĖĘō·¢£¬øĆĶ¬Ń§ĖłŌŚµÄ»ÆѧŠĖȤŠ”×éŌŚŹµŃéŹŅŃ”ÓĆFe(NO3)3ČÜŅŗĒåĻ“×ö¹żŅų¾µ·“Ó¦µÄŹŌ¹Ü£¬·¢ĻÖ²»µ«Ņų¾µČܽā£¬¶ųĒŅ½ĻÉŁ²śÉś“Ģ¼¤ŠŌĘųĢ唣
»ÆѧŠĖȤŠ”×é¶ŌFe(NO3)3ČÜŅŗČܽāŅųµÄŌĄķ½ųŠŠĢ½¾æ£ŗ
”¾Ģį³ö¼ŁÉč”æ¼ŁÉč1£ŗ Fe(NO3)3ČÜŅŗĻŌĖįŠŌ£¬ŌŚ“ĖĖįŠŌĢõ¼žĻĀNO3-ÄÜŃõ»ÆAg£»
¼ŁÉč2£ŗFe3+¾ßÓŠŃõ»ÆŠŌ£¬ÄÜŃõ»ÆAg
”¾ŃéÖ¤¼ŁÉč”æ
£Ø1£©¼×Ķ¬Ń§ŃéÖ¤¼ŁÉč1”£
¢ŁĖūÓƵ×ĻÉ«µÄFe(N03)3”¤9H20¾§Ģå£Ø·ÖĪö“棬Mr=404)ÅäÖĘ1.5mol/LµÄFe(N03)3ĀäŅŗ100mL”£ŠčŅŖ³ĘČ”_____g Fe(N03)3”¤9H20¾§Ģ壬ÅäÖĘ¹ż³ĢÖŠĖłÓƵ½µÄŅĒĘ÷³żÉÕ±”¢²£Į§°ōĶā»¹±ŲŠč£ŗ__________
¢Ś²āµĆ1.5 mol/LµÄFe(NO3)3ČÜŅŗpHŌ¼ĪŖ1£¬ĘäŌŅņÓĆ»ÆѧÓĆÓļ±ķŹ¾ĪŖ____”£
¢Ū½«pH=1µÄHN03ČÜŅŗ¼ÓČėµ½¶ĘÓŠŅų¾µµÄŹŌ¹ÜÖŠ£¬Õńµ“£¬¹Ū²ģµ½Ņų¾µĀżĀżČܽā£¬²śÉśĪŽÉ«ĘųĢå²¢ŌŚŅŗĆęÉĻ·½±äĪŖŗģ×ŲÉ«£¬ČÜŅŗÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½Ó¦ŹĒ_____
¢Ü½«1.5mol/LµÄFe(NO3)3ČÜŅŗ¼ÓČėµ½¶ĘÓŠŅų¾µµÄŹŌ¹ÜÖŠ£¬Õńµ“£¬¹Ū²ģµ½Ņų¾µŗÜæģČܽā£¬²¢ĒŅČÜŅŗŃÕÉ«¼ÓÉī”£
£Ø2£©ŅŅĶ¬Ń§ŃéÖ¤¼ŁÉč2”£·Ö±šÓĆČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ2%”¢10%µÄ×ćĮæFeCl3ČÜŅŗ¼ÓČėµ½¶ĘÓŠŅų¾µµÄŹŌ¹ÜÖŠ£¬Õńµ“£¬¶¼æ“²»³öŅų¾µČܽā”£ŅŅĶ¬Ń§ÓÉ“ĖµĆ³ö½įĀŪ£¬¼ŁÉč2²»³ÉĮ¢”£
ÄćŹĒ·ńĶ¬ŅāŅŅµÄ½įĀŪ?_______,¼ņŹöĄķÓÉ£ŗ_______
”¾Ė¼æ¼Óė½»Į÷”æI¼×Ķ¬Ń§µÄŹµŃé¢ÜÖŠ£¬ČÜŅŗŃÕÉ«ĪŖŹ²Ć“»į¼ÓÉī£æ²éŌÄ׏ĮĻµĆÖŖ£¬Fe2+ÄÜÓėNOŠĪ³ÉÅäĄė×Ó£ŗ £Ø×ŲÉ«)”£ŅŃÖŖ£¬Ķ¬ÅØ¶ČµÄĻõĖįŃõ»ÆŠŌ±ČFe3+ĀŌĒ攣
£Ø×ŲÉ«)”£ŅŃÖŖ£¬Ķ¬ÅØ¶ČµÄĻõĖįŃõ»ÆŠŌ±ČFe3+ĀŌĒ攣
øł¾ŻŅŌÉĻŠÅĻ¢×ŪŗĻ·ÖĪö£¬ÅØ”¢Ļ”Fe(N03)3ČÜŅŗČܽāŅų¾µŹ±£¬·¢ÉśµÄ·“Ó¦ÓŠŗĪ²»Ķ¬£æ
__________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĻÖÓŠŅ»ĘæŹµŃéŹŅ·ÅÖĆŅŃ¾ĆµÄæÉÄܱ»Ńõ»ÆµÄNa2SO3¹ĢĢ壬ĪŖĮĖŃŠ¾æĖüµÄ×é³É£¬ĒėÄć²ĪÓėĶ¬Ń§ĆĒ½ųŠŠµÄČēĻĀĢ½¾æ»ī¶Æ£ŗ
æÉŃ”ÓĆŹŌ¼Į£ŗÅØH2SO4”¢ÅØHNO3”¢10%ŃĪĖį”¢0.1mol/LH2SO4”¢0.1mol/LHNO3”¢0.1mol/LBaCl2”¢0.1mol/LBa(NO3)2”¢3%H2O2”¢10%NaOHČÜŅŗ”¢ÕōĮóĖ®”¢Ę·ŗģČÜŅŗ£»ŅĒĘ÷×ŌŃ””£
£Ø1£©Ģį³ö¼ŁÉč
¼ŁÉčŅ»£ŗ¹ĢĢåČ«²æŹĒNa2SO3£» ¼ŁÉ趞£ŗ¹ĢĢåČ«²æŹĒNa2SO4£»
¼ŁÉčČż£ŗ ”£
£Ø2£©Éč¼ĘŹµŃé·½°ø(ĀŌ)£»Ń”ÓĆĻĀĶ¼×°ÖĆ½ųŠŠŹµŃ飬øĆ×°ÖƵÄÓŵćŹĒ ”£
£Ø3£©½ųŠŠŹµŃé£ŗĒėŌŚĻĀ±ķÖŠÓĆ¼ņŅŖĪÄ×ÖŠ“³öŹµŃé²Ł×÷”¢Ō¤ĘŚĻÖĻóŗĶ½įĀŪ”£
| ŹµŃé²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč1£ŗČ”ŹŹĮæ¹ĢĢåѳʷӌĪ¢ŠĶŹŌ¹ÜÖŠ£»ŌŚW¹Üa“¦µĪČė ”¢b“¦µĪČė £»ÓĆ½ŗ¹Ü½«W¹ÜÓėĪ¢ŠĶŹŌ¹ÜĮ¬½ÓŗĆ | |
| ²½Öč2£ŗÓĆÕėĶ²ĪüČė £¬½«ÕėĶ·“©¹żĪ¢ŠĶŹŌ¹ÜµÄ½ŗČū£¬Ļņ¹ĢĢåŃłĘ·ÖŠ×¢ČėøĆČÜŅŗ”£ | ”£ |
| ²½Öč3£ŗ²¦³öÕėĶ²£¬ĪüČėÕōĮóĖ®Ļ“¾»£»ŌŁĪüČė ×¢ČėĪ¢ŠĶŹŌ¹ÜÖŠ | ”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĢśæóŹÆŹĒ¹¤ŅµĮ¶ĢśµÄÖ÷ŅŖŌĮĻÖ®Ņ»£¬ĘäÖ÷ŅŖ³É·ÖĪŖĢśµÄŃõ»ÆĪļ(ÉčŌÓÖŹÖŠ²»ŗ¬ĢśŌŖĖŲŗĶŃõŌŖĖŲ£¬ĒŅŌÓÖŹ²»ÓėĮņĖį·“Ó¦)”£Ä³ŃŠ¾æŠŌѧĻ°Š”×é¶ŌijĢśæóŹÆÖŠĢśµÄŃõ»ÆĪļµÄ»ÆѧŹ½½ųŠŠĢ½¾æ”£
¢ń.ĢśæóŹÆÖŠŗ¬ŃõĮæµÄ²ā¶Ø£¬ŅŌĻĀŹµŃé¹ż³Ģ²»ĶźÕū£¬Ēė²¹³äĶźÕū”£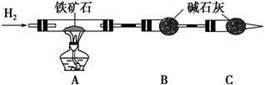
(1)°“ÉĻĶ¼×é×°ŅĒĘ÷£¬²¢______________________________________________£»
(2)½«8.0 gĢśæóŹÆ·ÅČėÓ²ÖŹ²£Į§¹ÜÖŠ£¬×°ÖĆB”¢CÖŠµÄŅ©Ę·ČēĶ¼ĖłŹ¾(¼Š³ÖŅĒĘ÷¾łŹ”ĀŌ)£»
(3)“Ó×ó¶Ėµ¼Ęų¹ÜæŚ“¦²»¶ĻµŲ»ŗ»ŗĶØČėH2£¬____________________________£¬
µćČ¼A“¦¾Ę¾«µĘ£»
(4)³ä·Ö·“Ó¦ŗ󣬳·µō¾Ę¾«µĘ£¬________________________________________£»
(5)²āµĆ·“Ó¦ŗó×°ÖĆBŌöÖŲ2.25 g£¬ŌņĢśæóŹÆÖŠŃõµÄ°Ł·Öŗ¬ĮæĪŖ________”£
¢ņ.ĢśæóŹÆÖŠŗ¬ĢśĮæµÄ²ā¶Ø£¬Į÷³ĢČēĻĀ”£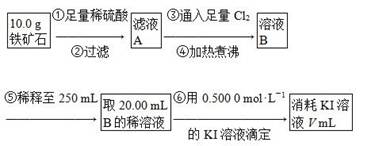
(1)²½Öč¢ÜÖŠÖ󷊵Ä×÷ÓĆŹĒ___________________________________________”£
(2)²½Öč¢ŻÖŠÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠÉÕ±”¢½ŗĶ·µĪ¹Ü”¢250 mLČŻĮæĘ攢________”£
(3)ĻĀĮŠÓŠ¹Ų²½Öč¢ŽµÄ²Ł×÷ÖŠĖµ·ØÕżČ·µÄŹĒ________”£
a£®ŅņĪŖµāĖ®ĪŖ»ĘÉ«£¬ĖłŅŌµĪ¶Ø¹ż³ĢÖŠ²»Šč¼ÓÖøŹ¾¼Į
b£®×¶ŠĪĘæ²»ŠčŅŖÓĆ“ż²āŅŗČóĻ“
c£®µĪ¶Ø¹ż³ĢÖŠæÉĄūÓƵķ·ŪČÜŅŗ×÷ÖøŹ¾¼Į
d£®µĪ¶Ø¹ż³ĢÖŠ£¬ŃŪ¾¦×¢ŹÓµĪ¶Ø¹ÜÖŠŅŗĆę±ä»Æ
e£®µĪ¶Ø½įŹųŗó£¬30 sÄŚČÜŅŗ²»»Öø“ŌĄ“µÄŃÕÉ«£¬ŌŁ¶ĮŹż
f£®µĪ¶Ø½įŹųŗ󣬵Ī¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ£¬Ōņ²ā¶Ø½į¹ūĘ«“ó
(4)ČōµĪ¶Ø¹ż³ĢÖŠĻūŗÄ0.500 0 mol”¤L£1 KIČÜŅŗ20.00 mL£¬ŌņĢśæóŹÆÖŠĢśµÄ°Ł·Öŗ¬ĮæĪŖ________”£
¢ó.ÓÉ¢ń”¢¢ņæÉŅŌĶĘĖć³öøĆĢśæóŹÆÖŠĢśµÄŃõ»ÆĪļµÄ»ÆѧŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ¹ż³Ģƻӊ·¢Éś»Æѧ·“Ó¦µÄŹĒ
| A£®ÓĆ»īŠŌĢæČ„³ż±łĻäÖŠµÄŅģĪ¶ |
| B£®ÓĆČČ¼īĖ®Ēå³ż“¶¾ßÉĻ²ŠĮōµÄÓĶĪŪ |
| C£®ÓĆ½žÅŻ¹żøßĆĢĖį¼ŲČÜŅŗµÄ¹čŌåĶĮ±£“ęĖ®¹ū |
| D£®ÓĆŗ¬¹č½ŗ”¢Ģś·ŪµÄĶøĘųŠ”“üÓėŹ³Ę·Ņ»ĘšĆÜ·ā°ü×° |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com