
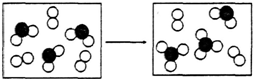
 A��B��C��D��E��F��ԭ��������������Ķ�����Ԫ�أ�B�Ƕ������н�������ǿ��Ԫ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ��������õİ뵼����ϣ�E���������������ڲ������֮��Ϊ3��5��A��Eͬ�壮
A��B��C��D��E��F��ԭ��������������Ķ�����Ԫ�أ�B�Ƕ������н�������ǿ��Ԫ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ��������õİ뵼����ϣ�E���������������ڲ������֮��Ϊ3��5��A��Eͬ�壮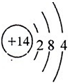
 ��
�� Al��OH��3��3H+���������������壬������ˮ�������ʹ֮���۴ﵽ��ˮĿ��
Al��OH��3��3H+���������������壬������ˮ�������ʹ֮���۴ﵽ��ˮĿ������ A��B��C��D��E��F��ԭ��������������Ķ�����Ԫ�أ�B�Ƕ������н�������ǿ��Ԫ�أ���B��NaԪ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ���C��AlԪ�أ�D���ʵľ��������õİ뵼����ϣ���DΪSiԪ�أ�E���������������ڲ������֮��Ϊ3��5��ԭ����3�����Ӳ㣬����������Ϊ6����EΪSԪ�أ���FΪClԪ�أ�A��Eͬ�壬��AΪOԪ�أ�
��1��DΪSiԪ�أ�ԭ�Ӻ�����3�����Ӳ㣬���������Ϊ2��8��7��
��2������������ȼ�����ɻ������ΪNa2O2��
��3����AlԪ�صĻ����ﳣ������ˮ������Ϊ��������ˮ���������������壬������ˮ�������
��4����ͼ�з�Ӧ���Ա�ʾΪ��A2+2BA2=2BA3��������������Ԫ����ɣ�Ӧ��Ϊ����������������Ӧ������������
��5����ҵ�Ͻ����������ͨ�����ڵĵ������п��Ƶû�����S2Cl2�������ʿ���ˮ��Ӧ����һ����ʹƷ����Һ��ɫ������ΪSO2��0.2mol�����ʲμӷ�Ӧʱת��0.3mol���ӣ���1molS2Cl2�μӷ�ӦҪת��1.5mol���ӣ�����ֻ��һ��Ԫ�ػ��ϼ۷����ı䣬��ӦΪ��Ԫ�صĻ��ϼ��ڸı䣬SԪ����+1�۽���Ϊ0�ۣ��ʷ�����ԭ��Ӧ��SΪ1.5mol���ٸ��ݵ���ת���غ����������������Ԫ�ػ��ϼۣ������жϲ�����д����ʽ��
��6��GΪ̼Ԫ�أ�����SԪ���γɵĻ�����CS2���������������ữ�ĸ������ˮ��Һ����������ǣ�ͬʱ�ų�CO2��ͬʱ��������ء���������ˮ��
��7������ZnS����ṹ��Si�ĵ��������ƣ���Si��������ʯ�ṹ���ƣ���Si����������4��̼ԭ�ӣ�����ԭ�Ӵ��ڶ��㡢���ģ����þ�̯�����㾧����Siԭ����Ŀ��������ZnS���徧����ԭ��������Si������ԭ��������ȣ��������㾧��������������ٸ��ݦ�=$\frac{m}{V}$���㣮
��� �⣺A��B��C��D��E��F��ԭ��������������Ķ�����Ԫ�أ�B�Ƕ������н�������ǿ��Ԫ�أ���B��NaԪ�أ�C�ǵؿ��к�����ߵĽ���Ԫ�أ���C��AlԪ�أ�D���ʵľ��������õİ뵼����ϣ���DΪSiԪ�أ�E���������������ڲ������֮��Ϊ3��5��ԭ����3�����Ӳ㣬����������Ϊ6����EΪSԪ�أ���FΪClԪ�أ�A��Eͬ�壬��AΪOԪ�أ�
��1��DΪSiԪ�أ�ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2������������ȼ�����ɻ������ΪNa2O2������ѧ�������Ӽ������ۼ��������ʽΪ ��
��
�ʴ�Ϊ�����Ӽ������ۼ��� ��
��
��3����AlԪ�صĻ����ﳣ������ˮ������Ϊ��������ˮ���������������壬������ˮ�������ʹ֮���۴ﵽ��ˮĿ�ģ���Ӧ�����ӷ���ʽΪ��Al3+��3H2O Al��OH��3��3H+��
Al��OH��3��3H+��
�ʴ�Ϊ�������ӷ���ˮ�⣺Al3+��3H2O Al��OH��3��3H+���������������壬������ˮ�������ʹ֮���۴ﵽ��ˮĿ�ģ�
Al��OH��3��3H+���������������壬������ˮ�������ʹ֮���۴ﵽ��ˮĿ�ģ�
��4����ͼ�з�Ӧ���Ա�ʾΪ��A2+2BA2=2BA3��������������Ԫ����ɣ�Ӧ��Ϊ����������������Ӧ�����������÷�Ӧ����ʽΪ��O2+2SO2$\frac{\underline{����}}{��}$2SO3��
�ʴ�Ϊ��O2+2SO2$\frac{\underline{����}}{��}$2SO3��
��5����ҵ�Ͻ����������ͨ�����ڵĵ������п��Ƶû�����S2Cl2�������ʿ���ˮ��Ӧ����һ����ʹƷ����Һ��ɫ������ΪSO2��0.2mol�����ʲμӷ�Ӧʱת��0.3mol���ӣ���1molS2Cl2�μӷ�ӦҪת��1.5mol���ӣ�����ֻ��һ��Ԫ�ػ��ϼ۷����ı䣬��ӦΪ��Ԫ�صĻ��ϼ��ڸı䣬SԪ����+1�۽���Ϊ0�ۣ��ʷ�����ԭ��Ӧ��SΪ1.5mol����������SΪ1mol��2-1.5mol=0.5mol��������������SԪ�ػ��ϼ�Ϊa����0.5mol����a-1��=1.5�����a=4�������ɶ������÷�Ӧ�Ļ�ѧ����ʽΪ��2S2Cl2��2H2O=3S+SO2��+4HCl��
�ʴ�Ϊ��2S2Cl2��2H2O=3S+SO2��+4HCl��
��6��aΪ̼Ԫ�أ�����SԪ���γɵĻ�����CS2���������������ữ�ĸ������ˮ��Һ����������ǣ�ͬʱ�ų�CO2��ͬʱ��������ء���������ˮ����ƽ��ѧʽ����ʽΪ��5CS2+4KMnO4+6H2SO4=2K2SO4+4MnSO4+10S��+5CO2��+6H2O��
�ʴ�Ϊ��5CS2+4KMnO4+6H2SO4=2K2SO4+4MnSO4+10S��+5CO2��+6H2O��
��7������ZnS����ṹ��Si�ĵ��������ƣ���Si��������ʯ�ṹ���ƣ���Si����������4��̼ԭ�ӣ�����ԭ�Ӵ��ڶ��㡢���ģ��ʾ�����Siԭ����ĿΪ4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��������ZnS���徧���к���4��Zn��4��Sԭ�ӣ���������Ϊ4��$\frac{87}{{N}_{A}}$g�������߳�Ϊacm���������Ϊ��acm��3�����ܶ�Ϊ4��$\frac{87}{{N}_{A}}$g�£�acm��3=$\frac{4��87}{{a}^{3}{N}_{A}}$g•cm3��
�ʴ�Ϊ��$\frac{4��87}{{a}^{3}{N}_{A}}$��
���� ���⿼��ṹ����λ�ù�ϵ�ۺ�Ӧ�ã���Ŀ�Ƚ��ۺϣ��漰��������Ų�������ʽ������ˮ��Ӧ�á�������ԭ��Ӧ����������ȣ���7���о�������Ϊ�״��㡢�ѵ㣬��Ҫѧ�������ѧ�����ľ����ṹ���ѵ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaCl2��Һ | B�� | K2SO4��Һ | C�� | CuSO4 | D�� | NH4NO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɺ������֪�����л��������������ֲ�ͬ�Ļ�ѧ�� | |
| B�� | �ɺ˴Ź�������֪�����л�������������ֲ�ͬ��ѧ��������ԭ�� | |
| C�� | ������˴Ź�����������֪������е���ԭ������ | |
| D�� | ��A�Ļ�ѧʽΪC3H6O������ṹ��ʽΪCH3COCH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | F- | B�� | NH3 | C�� | H2O | D�� | Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��OH��2 | B�� |  | C�� | NaAlO2 | D�� | Na2SiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ��ʯ�������ǿ�������Դ | |
| B�� | ú��������Һ���������������仯���̣��ɱ�Ϊ�����Դ | |
| C�� | PM 2.5���е�Ǧ��������ȶ������к���Ԫ�ؾ����ؽ���Ԫ�� | |
| D�� | ҽҩ�г��þƾ�������������Ϊ�ƾ��ܹ�ʹϸ�������巢������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ô�������β���е�CO��NO��CO+NO$\frac{\underline{\;����\;}}{\;}$C+NO2 | |
| B�� | ����SO2ͨ�뵽Ba��OH��2��Һ��SO2+Ba2++2OH-�TBaSO3��+H2O | |
| C�� | NH4HCO3���ڹ�����NaOH��Һ�У�HCO3+OH-�TCO32-+H2O | |
| D�� | Ũ������������̹��ȣ�Mn2O+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++2Cl-+Cl2��+2H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com