
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ�仹ԭ����ΪMn2+������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ���������ʾ��
�ٳ�ȡ�Զ�����������KMnO4��������ˮ�У�����Һ���Ȳ�������1 h�������ײ���©�����˳�ȥ���ܵ�MnO(OH)2���۹��˵õ�KMnO4��Һ�����棻������������ԭ�ζ���������70��80 ���������û��Լ�(���ȸߡ���Է��������ϴ��ȶ��ԽϺõ�����)��Һ�궨��Ũ�ȡ�
��ش��������⣺
(1)���ƺõ�KMnO4��Һ���淽���� ��ȷ��ȡһ�������KMnO4��Һ��Ҫʹ�õ�������____________��
(2)�����������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ��________(�����)��
| A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
(1) ��������ɫ�Լ�ƿ�����ڰ��� ��ʽ�ζ��� (2)A (3) 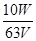 ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
�������������(1) ����KMnO4�����ֽ⣬����KMnO4��Һ���淽������������ɫ�Լ�ƿ�����ڰ���������KMnO4��Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ����¶˵���Ƥ�ܲ����ü�ʽ�ζ�����ȡ��Ӧ����ʽ�ζ�����ȡ��(2) ����FeSO4��Na2SO3�ڿ��������������ʣ�Ũ�����ӷ����궨�궨KMnO4��ҺŨ��ʱ��������H2C2O4��2H2O �����ȶ�����ѡA����3��H2C2O4��KMnO4��Һ��Ӧ��H2C2O4������ΪCO2��KMnO4����ԭΪMn2+�����ݵ����غ�Ĺ�ϵʽ��5H2C2O4����2KMnO4����������ݴ������ɵ�KMnO4��Һ�����ʵ���Ũ��Ϊ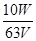 mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
���㣺����ҩƷ�ı��桢�ζ�ʵ�����ӷ���ʽ��д��ϵʽ�����㡣


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ��
��1�������й�������ȷ����__________����д��ţ�
a��ʹ��������ƽ�ĵ�һ�������ǽ��������������̶ȴ�
b�����˲��������У�Ϊ�ӿ�����ٶȿ��ò�������©���е���Һ���н���
c����Ũ��������ϡ��Һʱ������Ͳ�к�ϡ��Ҫ��ȴ��������ת�Ƶ�����ƿ��
d��������ƿ������Һʱ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��ߴ���������ҺŨ��ƫ��
��2�������������壺H2��O2��NH3��SO2��NO2��NO������������ͼ��ʾװ�ý����ռ���
���������B�ڽ��룬���ռ���������_______________��
��������ƿ��ע��ˮ��������Ӧ�ô�___����д��A����B�����ڽ��룬�����ռ���������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѧʵ���ڻ�ѧѧϰ�о�����Ҫ�����á�
��1��������ʵ���йص�������ȷ���� (�����)��
�ٰ�ˮ��Ũ������廯����������������ĥ��������ɫ����ƿ��
������ƿ����Һ©������(��)ʽ�ζ��ܵ�������ʹ��ǰ����������Ƿ�©ˮ
����ϡ���ἴ�ɼ���Na2SiO3��NaHCO3��Na[Al(OH)4]��Na2SO4������Һ
���������Ҵ��Ļ��Һ���÷�Һ©�����з���
����������ƽȷ��ȡ29��25gNaCl���壬����500mL0��5mol��L-1 NaCl��Һ
��2��ʵ������Fe2O3��CO��Ӧ����ȡ����Fe��
���밴��������������������װ��(ÿ��װ��ֻ����ʹ��һ��)��������˳��ΪA ��
��
��װ��C�������� ��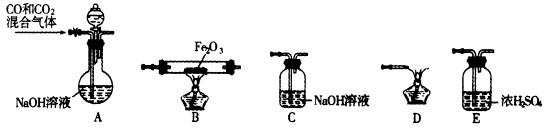
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֡�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ�����������ϸߵ������ӣ�Ȼ������ת��Ϊ��ͨ�����ӣ�����������Ժ��ų�����ʵ�����õ�������������ͼ��
����Ҫ����д���пհף�
��1����װ��������װ��ʱ��Ӧѡ�õ�����������Ϊ (��дͼ�б��)��
��2����ʵ�����ʱ����������������ҵ�˳�����������ĸ����������ܵı������Ϊ ��
��3�������ٵ���Ƥ��������Ӧ��2����ԭ���� ��
��4����ʵ��������10mol·L��1��NaOH��Һ500mL���õ�����������������ƽ���ձ��⣬�����õ��������� (����������) ������ʱ������ͼ����������ҺŨ�� (�ƫ�ߡ���ƫ�͡�)��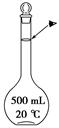
��5��ʵ��ʱ��������ClO����H2O2��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᡣijѧϰС���ͬѧ���Ը�����Ϊԭ����ˮ�⡪������ˮ��ѭ��������ȡ���ᡣ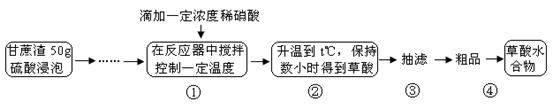
|
|
 �����������Ϣ�ش��������⣺
�����������Ϣ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�������������������������������ʡ�����ʵ���������£�
��ش��������⣺
��1������1���õ��IJ���������������____________��
��2������Һ3��ȡ�������������ӷ���ʽΪ ��
��3������д����֤��Һ1����NH4+��ʵ����̣�_______________________________________________��
��4��ʵ������Fe2O3��CO��Ӧ����ȡ����Fe��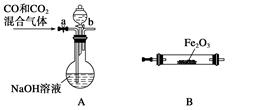

���밴���������ҵķ����������и�װ�ã�˳��ΪA�� ��
�ڼ��װ��A�����Եķ����� ��
���ڵ�ȼB���ľƾ���ǰ��Ӧ���еIJ�����_______________________________________����װ��C��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺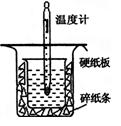
(1)����ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��_________ _������֮�⣬װ���е�һ�����Դ����� ��
(2)Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������________ ___��
(3)������60mL 0.25mol��L-1 H2SO4��50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ������ʵ����ȣ����ų������� �����ȡ���������ȡ�������ʵ���������ȷ���������к��� ���ȡ�������ȡ���
(4)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز�������������
B����������������
C��һ��Ѹ�ٵ���
(5)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ���
B���ҿ�ӲֽƬ�ò���������
C����������ձ�
D���������¶ȼ��ϵĻ��β���������ؽ���
(6)ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijNa2CO3��Ʒ�л���һ������Na2SO4 (��������ᾧˮ����ij��ѧ��ȤС��������ַ����ⶨ����Ʒ��Na2CO3�������������Իش��������⡣
����һ���������з������Na2CO3���������Ĝy��
(1)�����ۺܵ͢����Ʒֱ�Ϊ_______��
(2)���������١����У�ʹ�õ�����������______(��������)��
(3)�жϲ����ڷ���ɵķ�����______
��������������ͼʵ��װ�ã��г�������ʡ�ԣ�.ѡ�������Լ�: a.Ũ����b.����NaHCO3��ҺC.6mol/L����D.2mol/L����, e.��ʯ��f. ��ˮCaCl2,�y����Ʒ��Na2CO3,������������
(4)��д���пո�
| ���� | �Լ� | ������Լ���Ŀ�� |
| A | | �������ʱϴȥCO2 |
| B | | ʹ��Ʒ��ַ�Ӧ�ų����� |
| C | a | |
| D | e | �������CO2 |
| E | e | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���
ͼ1 ͼ2 ͼ3 ͼ4
| A����ͼ1��ʾװ�ó�ȥCl2�к��е�����HCl |
| B����ͼ2��ʾװ������NH4Cl������Һ�Ʊ�NH4Cl���� |
| C����ͼ3��ʾװ����ȡ����������CO2���� |
| D����ͼ4��ʾװ�÷���CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com