
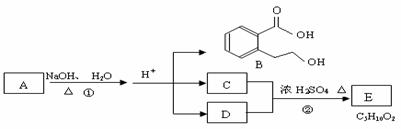
 ĻĀĶ¼ÖŠ A”¢B”¢C”¢D”¢E¾łĪŖÓŠ»ś»ÆŗĻĪļ”£ŅŃÖŖ£ŗCÄÜøśNaHCO3·¢Éś·“Ó¦£»£ĆŗĶ£ÄµÄĻą¶Ō·Ö×ÓÖŹĮæĻąµČ£¬ĒŅEĪŖĪŽÖ§Į“µÄ»ÆŗĻĪļ”£øł¾ŻĻĀĶ¼»Ų“šĪŹĢā£ŗ
ĻĀĶ¼ÖŠ A”¢B”¢C”¢D”¢E¾łĪŖÓŠ»ś»ÆŗĻĪļ”£ŅŃÖŖ£ŗCÄÜøśNaHCO3·¢Éś·“Ó¦£»£ĆŗĶ£ÄµÄĻą¶Ō·Ö×ÓÖŹĮæĻąµČ£¬ĒŅEĪŖĪŽÖ§Į“µÄ»ÆŗĻĪļ”£øł¾ŻĻĀĶ¼»Ų“šĪŹĢā£ŗ
£Ø1£©C·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ ______________£»»ÆŗĻĪļB²»ÄÜ·¢ÉśµÄ·“Ó¦ŹĒ £ØĢī×ÖÄøŠņŗÅ£©£ŗ
a ¼Ó³É·“Ó¦ bČ”“ś·“Ó¦ cĻūČ„·“Ó¦ dõ„»Æ·“Ó¦ eĖ®½ā·“Ó¦ f ÖĆ»»·“Ó¦
£Ø2£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ_____ _”£
£Ø3£©·“Ó¦¢ŚŹµŃéÖŠ¼ÓČȵÄÄæµÄŹĒ£Ø“ÓĖŁĀŹŗĶĘ½ŗāĮ½øö½Ē¶Č·ÖĪö£©
(4) AµÄ½į¹¹¼ņŹ½ŹĒ __________________ ”£
£Ø5£©Ķ¬Ź±·ūŗĻĻĀĮŠČżøöĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹ĢåµÄŹżÄæÓŠ øö”£
I.ŗ¬ÓŠ¼ä¶žČ”“ś±½»·½į¹¹ ¢ņ.ŹōÓŚ·Ē·¼ĻćĖįõ„ ¢ó.Óė FeCl3 ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦”£
Š“³öĘäÖŠČĪŅāŅ»øöĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ŅŅŌ×ÓÓŠČżøöµē×Ó²ć£¬µŚŅ»²ćÓė×īĶā²ćµē×ÓŹżĻąµČ£»¼×Ō×ÓµÄŗĖĶāµē×ÓŹż±ČŅŅŌ×ÓŗĖĶāµē×ÓŹżÉŁ1£»±ūŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµē×ÓŹżµÄ2±¶£»¶”Ō×ÓŗĖµēŗÉŹż±Č±ūŌ×ÓŗĖµēŗÉŹż¶ą2”£Ēė»Ų“š£ŗ
£Ø1£©¼×ŹĒ ŌŖĖŲ£ØĢīŌŖĖŲ·ūŗÅ£©£¬¼×µÄµ„ÖŹÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ:
£»
£Ø2£©ŅŅŹĒ ŌŖĖŲ£ØĢīŌŖĖŲ·ūŗÅ£©£¬Ō×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ______________________£»
£Ø3£©±ūŹĒ ŌŖĖŲ£ØĢīŌŖĖŲĆū³Ę£©£¬ĖüŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆ £»
£Ø4£©¶”ŹĒ ŌŖĖŲ£ØĢīŌŖĖŲĆū³Ę£©£¬¼×Óė¶”Į½ÖÖŌŖĖŲæÉ×é³ÉµÄĪļÖŹÓŠ
”¢ ”££ØĢī»ÆѧŹ½£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°“ĻĀĶ¼ŹµŃé×°ÖĆ½ųŠŠŹµŃ飬»Ų“šĻĀĮŠĪŹĢā£ŗ£Ø¹²10·Ö£©
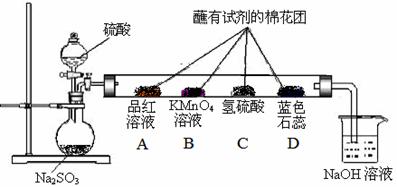
£Ø1£©ÓĆÓŚÓėNa2SO3×÷ÓƵÄĮņĖįŅŖŹ¹ÓĆ £ØĢī”°Ļ”ĮņĖį”±»ņ”°ÅØĮņĖį”±£©
£Ø2£©ÉÕ±ÖŠNaOHČÜŅŗŌŚŹµŃéÖŠµÄ×÷ÓĆŹĒ £»
·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©Ö±ŠĪŹŌ¹ÜÖŠø÷“¦¹Ū²ģµ½µÄĻÖĻó·Ö±šŹĒ£ŗA £»B £»C £»D ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼ŗĻ©“Ę·ÓŹĒČĖ¹¤ŗĻ³ÉµÄ¼¤ĖŲĄąŅ©Īļ£¬Ęä½į¹¹ČēĻĀ”£ĻĀĮŠÓŠ¹ŲŠšŹö²»ÕżČ·µÄŹĒ£Ø £©
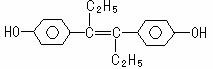
A.¼ŗĻ©“ʷӵķÖ×ÓŹ½ĪŖC18H20O2
B.¼ŗĻ©“Ę·ÓæÉÓėNaOHŗĶNaHCO3·¢Éś·“Ó¦
C.1 moløĆÓŠ»śĪļæÉŅŌÓė5 mol Br2·¢Éś·“Ó¦
D.øĆÓŠ»śĪļ×ī¶ąæÉÄÜÓŠ18øöĢ¼Ō×Ó¹²Ćę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖAĪļÖŹµÄ·Ö×Ó½į¹¹¼ņŹ½ČēĶ¼£¬1mol AÓė×ćĮæµÄNaOHČÜŅŗ»ģŗĻ¹²ČČ£¬³ä·Ö·“Ó¦ŗó×ī¶ąĻūŗÄNaOHµÄĪļÖŹµÄĮæĪŖ£Ø””””£©
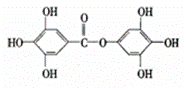
| ”” | A£® | 6mol | B£® | 7mol | C£® | 8mol | D£® | 9mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅÖʱøŃõĘųµÄ·½·Øŗܶą”£ĻÖŌŚŅ»¶ØĢõ¼žĻĀ£¬ĄūÓĆ¹żŃõ»ÆĒā”¢ĀČĖį¼ŲŗĶøßĆĢĖį¼ŲĪŖ·“Ó¦ĪļÖʱøŃõĘų£¬ŌŚĶ¬ĪĀĶ¬Ń¹ĻĀ£¬ÖĘČ”µÄŃõĘųĢå»żĻąĶ¬Ź±£¬Čżøö·“Ó¦ÖŠ×ŖŅʵĵē×ÓŹżÖ®±ČĪŖ(””””)
A£®2”Ć2”Ć1 B£®1”Ć2”Ć2 C£®2”Ć1”Ć2 D£®1”Ć1”Ć1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÖŠ£¬ŹōÓŚĖ®½ā·“Ó¦µÄŹĒ
A£®HCOOH+H2O HCOO£ + H3O+ B£® HS£ + H2O
HCOO£ + H3O+ B£® HS£ + H2O S2£ + H3O+
S2£ + H3O+
C£®CO32£ + H2O HCO3£ + OH£ D£®HCO3£+H2O
HCO3£ + OH£ D£®HCO3£+H2O CO3 2£ + H3O+
CO3 2£ + H3O+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ¶ĢÖÜĘŚŌŖĖŲµÄĄė×ÓaA3+”¢bB+”¢cC2-”¢dD-¶¼¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹,ŌņĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ””(””””)
A.Ō×Ó°ė¾¶:A>B>D>C
B.Ō×ÓŠņŹż:d>c>b>a
C.Ąė×Ó°ė¾¶:C>D>B>A
D.µ„ÖŹµÄ»¹ŌŠŌ:A>B>D>C
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com