
���� ��1��Crԭ�Ӻ��������Ϊ24������������ԭ�������ع���������д����Cr3+�γ���λ�������������ˮ���ӣ�
��2������������-COO-��Cԭ���γ�3���Ҽ�������̼ԭ���γ�4���Ҽ�����û�й¶Ե��ӣ��ӻ������Ŀ�ֱ�Ϊ3��4��
��3��ԭ����Ŀ��ȡ��۵�������Ҳ��ȵ�����Ϊ�ȵ����壻�������������֮���γ������
��� �⣺��1��Crԭ�Ӻ��������Ϊ24����������Ų�ʽΪ1s22s22p63s23p63d54s1�������Ӵ�����磬��Cr3+�γ���λ�������������H2O���ӣ�
�ʴ�Ϊ��1s22s22p63s23p63d54s1��H2O��
��2������������-COO-��Cԭ���γ�3���Ҽ�������̼ԭ���γ�4���Ҽ�����û�й¶Ե��ӣ��ӻ������Ŀ�ֱ�Ϊ3��4���ֱ��ȡsp2��sp3�ӻ���
�ʴ�Ϊ��sp2��sp3�ӻ���
��3��ԭ����Ŀ��ȡ��۵�������Ҳ��ȵ�����Ϊ�ȵ����壬��H2O��Ϊ�ȵ������һ�ַ���ΪH2S��NH3��H2O�е��ܽ�Ⱥܴ���Ϊ���Ƕ��Ǽ��Է����⣬����Ϊ�������������֮���γ������
�ʴ�Ϊ��H2S���������������֮���γ������
���� ���⿼���������Ų����ӻ���ʽ�жϡ��ȵ����塢�����ע��Ժ��ع������������⣬ע��������������ʵ�Ӱ�죮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
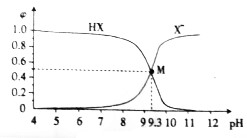
| A�� | 25��ʱ��$\frac{c��HX��}{c��{X}^{-}��•c��{H}^{+}��}$=109.3 | |
| B�� | M����Һ�д��ڣ�c��X-��+c��OH-��=c��H+��+c��Na+�� | |
| C�� | �μ�����Ĺ����У�ˮ�ĵ���̶��ȼ�С������ | |
| D�� | �����£���Ũ�ȵ������HX��NaX��Һ��Ϻ���Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͬ�������ͬpH������һԪ���зֱ��������п�ۣ�����������������������ԱȽ�����һԪ���������Խ��� | |
| B�� | ��ʢ��1mL��������Һ���Թ��еμ�NaCl��Һ����ǡ�ò����г������ɣ��������еμ�Na2S��Һ�����ɺ�ɫ������˵��һ�ֳ�����ת��Ϊ��һ���ܽ�ȸ�С�ij��� | |
| C�� | �����£���pH��ֽ�ⶨŨ��Ϊ0.1mol/LNaClO��Һ��0.1mol/LCH3COONa��Һ��pH�����ԱȽ�HClO��CH3COOH������ǿ�� | |
| D�� | �ڵ��з�̪Na2CO3��Һ����μ���BaCl2��Һ����ɫ����ȥ��˵��Na2CO3��Һ�д���ˮ��ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
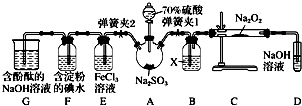
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���еĵ�����ͬһ�������˶� | |
| B�� | �����ߵĵ�������˽��������˶��������͵ĵ��������Զ�������˶� | |
| C�� | �������������ԭ�ӽл�̬ԭ�� | |
| D�� | ͬһԭ���У�1s��2s��3s�������ɵĵ�����Խ��Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| I1 | I2 | I3 | I4 | I5 | �� | |
| R | 740 | 1500 | 7700 | 10500 | 13600 | �� |
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢۢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
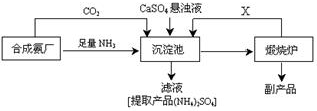
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com