
 CO2(g)+H2(g) ������COת����H2������ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ���õ�������������
CO2(g)+H2(g) ������COת����H2������ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ���õ�������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����һ�������£�������Ӧ�ﵽƽ�⣬��������̼����������̼����Ԫ�ص�������m(C)��m(O)����Ϊ__________����ѡ���
A.3��1 B.3��4 C.3��5 D.3��8
��2����CO��ת����Ϊ75%����̼����������m(C)��m(O)Ϊ_________��
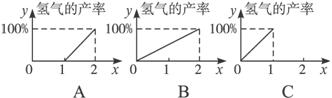
��3������COx��ʾƽ��������̼�����������ɣ����ʾx�뷴ӦCO+H2O��g��![]() CO2+H2��H2���ʹ�ϵ��ȷ����________����ͼ����ţ���
CO2+H2��H2���ʹ�ϵ��ȷ����________����ͼ����ţ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com