
 ��
�� ��
�� ��
��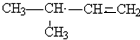 ������һ�֣�����д�����е�һ�֣�
������һ�֣�����д�����е�һ�֣����� ��1�����������ΪCxHy����ͬ���ʵ���������������ȡ���ڣ�x+$\frac{y}{4}$������x+$\frac{y}{4}$����ֵԽ�������Խ��
���顢��ϩ����Ȳ�ķ���ʽ�ֱ�ΪC2H6��C3H6��C4H6�������к��е�Hԭ����Ŀ��ͬ��������Ԫ���غ��֪�����ʵ�����ͬ����ˮ��������ͬ��
1molij�����������Ժ�1molCl2�����ӳɷ�Ӧ������2��3-����-2-�����飬���ݼӳɷ�Ӧ��ԭ������ȡ���Ʒ���ԭC=C˫�����ӷ����м�����ԭ���γ�C=C˫�����ɵò��������Ľṹ���ٸ���ϵͳ����������������
��2������ȼ�����ɶ�����̼��ˮ����0.2mol��ȼ������B��C��������̼��ˮ��1mol������C��H�غ��жϷ���ʽ��
������A����ʹ��ˮ��ɫ������һ�������£�����Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�˵�����в���̼̼˫����ӦΪ���������������飻
����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���3������˵�����к���C=C�����к���3��������3�֣��Դ�ȷ��ϩ����
��3���ױ��ɱ����Ը�������������Ҵ�������ˮ�����������������Һ��Ӧ��
��� �⣺��1���������ʽΪC2H6���䣨x+$\frac{y}{4}$��=2+$\frac{6}{4}$=3.5����ϩ�ķ���ʽΪC3H6���䣨x+$\frac{y}{4}$��=3+$\frac{6}{4}$=4.5����Ȳ�ķ���ʽΪC4H6���䣨x+$\frac{y}{4}$��=4+$\frac{6}{4}$=5.5������ͬ���ʵ�����������Ȳ�ĺ�������ࣻ
���顢��ϩ���ķ���ʽ�ֱ�ΪC2H6��C3H6��C4H6�������к��е�Hԭ����Ŀ��ͬ��������Ԫ���غ��֪�����ʵ�����ͬ����ˮ��������ͬ��
1molij�����������Ժ�1molCl2�����ӳɷ�Ӧ������2��3-����-2-�����飬���ݼӳɷ�Ӧ��ԭ������ȡ���Ʒ���ԭC=C˫�����ӷ����м�����ԭ���γ�C=C˫�����ɵò��������ĽṹΪ��CH3��2C=CH-CH2-CH3������ϵͳ����������Ϊ2-��-2-��ϩ��
�ʴ�Ϊ����Ȳ�� ˮ��C��CH3��2=CHCH2CH3��2-��-2-��ϩ��
��2����ij��A 0.2mol ����������ȫȼ�պ�����B��C��CO2��H2O��1mol��������к���N��C��=5��n��H��=10������ʽΪC5H10���ʴ�Ϊ��C5H10��
������A����ʹ��ˮ��ɫ������һ�������£�����Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�˵�����в���̼̼˫����ӦΪ���������������飬�ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���3������˵�����к���C=C�����к��� 3��������3�֣���A�����еĽṹ��ʽΪ ��
�� ��
��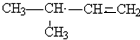 ��
��
�ʴ�Ϊ�� ��
�� ��
��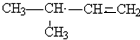 ��
��
��3���ױ��ɱ����Ը���������������ɵı����������ˮ���Ҵ�������ˮ���ɼ�ˮ���룬���������������Һ��Ӧ��Ȼ��ֲ���룬
�ʴ�Ϊ������KMnO4��Һ�� ˮ�� NaOH��Һ��
���� ���⿼���л�����ƶϣ��������л���Ľṹ�����ʵĿ��飬Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ����������ͬ���칹����жϡ��л���Ӧ�ļ����Լ�����ע��������ʵ������жϿ��ܾ��еĽṹ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢܢ٢� | B�� | �ڢ٢ܢ� | C�� | �٢ڢۢ� | D�� | �٢ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ڼ���ʱ����ȡ����Ӧ���ױ��������ڳ����¾��ܷ���ȡ����Ӧ | |
| B�� | �Ҵ������Ӷ����ǻ������ǵ��볣����ͬ | |
| C�� | �������������������ˮ����ȡ����Ӧ | |
| D�� | 1 mol�ױ�����3 mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��������е�4����ԭ�Ӷ����Ա�ȡ��������������е�4����ԭ�Ӷ�������ȡ�������Եõ��ķ�����ͼ���Ը÷��ӵ�����������ȷ���ǣ�������
��������е�4����ԭ�Ӷ����Ա�ȡ��������������е�4����ԭ�Ӷ�������ȡ�������Եõ��ķ�����ͼ���Ը÷��ӵ�����������ȷ���ǣ�������| A�� | ����ʽΪC25H20 | B�� | ���е�̼ԭ�ӿ��ܶ���ͬһƽ���� | ||
| C�� | ����ԭ��һ����ͬһƽ���� | D�� | ���������ڷ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol H2O��D2O��ɵĻ�����к�����������NA | |
| B�� | 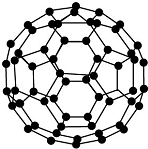 28 g N60�����ӽṹ��ͼ��ʾ���к��е�N-N����ĿΪ1.5NA | |
| C�� | 0�桢1.01��105 Paʱ��11.2 L������������ԭ����ԼΪNA | |
| D�� | �����£���5.6 g��Ͷ��������ϡ�����У���ַ�Ӧ��ת�Ƶ�����Ϊ0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
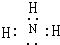 ��ʵ������ȡ���⻯��Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��ʵ������ȡ���⻯��Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com