
���� ��1�������Σ���ɫ��Ӧ���������ɫ�ʻ�ɫ���ݴ���֤Na2SO3�����Σ�
�����Թ���ȡ����Na2SO3���壬����75%��������Һ���Ƶö���������������ʹƷ����ɫ�����������Ư������ʱ�ģ����Ⱥ��ָ�ԭɫ���ݴ˲�����ȫʵ�������
��2���������������ʣ���Һ�л���������ƣ����ù��������ȥ����������ӣ�Ȼ�����Ȼ��������Ƿ�����������ӣ��Ӷ��ж����������Ƿ���ʣ�
��3�����нᾧˮ�ľ��壬��������ʧȥ�ᾧˮ��������Ļ��ϼ۷�������һ������ΪNa2SO4���ݴ���д����ʽ��
��� �⣺��1���ٽ������������ɫ��Ӧ���ֵ�ɫ�ʷḻ�������������Σ��������ɫ�ʻ�ɫ����֤Na2SO3�����Σ���������ɫ��Ӧʵ�飬
�ʴ�Ϊ����ɫ��Ӧ��
�ڸ���ǿ���������ԭ�������������ǿ�������ᣬNa2SO3�������Ũ�����ᣨԼ75%����Ӧ����ѧ��Ӧ����ʽΪ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����������ͨ�뵽Ʒ����Һ�У�Ʒ����ɫ��������Һ���ָֻ�Ϊԭ����ɫ��˵��������ΪSO2��
�ʴ�Ϊ��������ͨ�뵽Ʒ����Һ�У�Ʒ����ɫ��������Һ���ָֻ�Ϊԭ����ɫ��
��2������������Һ�ڿ������ױ��ʣ��������������ƣ�����Һ�л������������ӣ������ж�����������Һ�Ƿ���ʵķ���Ϊ��ȡ�����������Թܣ���ˮ�ܽ⣬�μӹ���������������ų����������ɣ��ٵμ�BaCl2���а�ɫ��������֤�������Ѿ����ʣ�
�ʴ�Ϊ��ȡ������Һ���Թ��У���������ϡ���ᣬ�������ɣ��ټ���BaCl2��Һ���а�ɫ�������ɣ�����Һ���ʣ�
��3��25.2g������ Na2SO3•7H2O�����ڸ����¸����������������أ���ȴ��Ƶù���Ϊ12.6g��
��n��Na2SO3•7H2O��=$\frac{25.2g}{252g/mol}$=0.1mol�����ɵ����������Ϊ25.2-12.6=12.6g�����нᾧˮ�ľ��壬��������ʧȥ�ᾧˮ��
�����ɵ���ˮ������n��H2O��=$\frac{12.6g}{18g/mol}$=0.7mol����ǡ������Na2SO3•7H2O��ȡ��Ӧ���������������ˮ�����Һ�������飬��Һ�к���S2-����˵������Na2S�����ݻ��ϼ۵ı仯��֪����һ������ΪNa2SO4����Na2SO3�ڸ�����������������������ԭ��Ӧ��4Na2SO3$\frac{\underline{\;����\;}}{\;}$Na2S+3Na2SO4��
�ʴ�Ϊ��4Na2SO3$\frac{\underline{\;����\;}}{\;}$Na2S+3Na2SO4��
���� ���⿼���˺���������ʣ��漰���Ӽ��顢������ԭ��Ӧ������ʽ����д�ȣ���Ŀ�Ѷ��еȣ�ע�����仯��������ʣ�


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3 ��Һ�У�c��H+��+c��H2CO3���Tc��CO32-��+c��OH-�� | |
| B�� | 0.1 mol/L��������Һ20 mL��0.1 mol/L����10 mL��Ϻ����Һ�У�c��CH3COO-����c��Cl-����c��H+����c��CH3COOH�� | |
| C�� | ���ʵ���Ũ����ȵĢ�NH4Cl���ڣ�NH4��2SO4����NH4Al��SO4��2������Һ�У�c��NH4+�� �ɴ�С��˳��Ϊ�ۣ��ڣ��� | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol/L�Ģٰ�ˮ����NaOH��Һ����Na2CO3��Һ����NaHCO3��Һ��pH�Ĵ�С˳�ڣ��ۣ��ܣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al2O3�Ȳ���������Ҳ���ǻ�ԭ�� | B�� | Cl2����ԭ | ||
| C�� | ÿ����1 mol CO2ת��2 mol���� | D�� | CO2Ϊ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ǽ����ԣ�Cl��Br | B�� | ���ԣ�H2SO4��H3PO4 | ||
| C�� | ���ԣ�KOH��NaOH | D�� | ���ȶ��ԣ�Na2CO3��NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
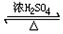 CH3COOC2H5+H2O ��������Ũ���������������ȣ�
CH3COOC2H5+H2O ��������Ũ���������������ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
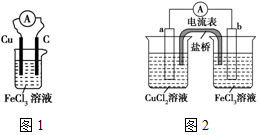 ʵ���������²��ϣ�ͭƬ����Ƭ��ʯī����CuCl2��Һ��FeCl3��Һ�����ߡ������������ţ�װ����֬-KCl��U�ιܣ����ձ��ȣ���ͬѧ�������ͼ1��ԭ���װ�ã�����ͬѧ���ּ�ͬѧ��Ƶ�ԭ���װ��Ч�ʲ��ߣ������ڶ�ʱ���ھͻ�˥����Ϊ����������⣬��ԭ�����Ƴ��˴����ŵ�װ�ã���ͼ2��ʾ��
ʵ���������²��ϣ�ͭƬ����Ƭ��ʯī����CuCl2��Һ��FeCl3��Һ�����ߡ������������ţ�װ����֬-KCl��U�ιܣ����ձ��ȣ���ͬѧ�������ͼ1��ԭ���װ�ã�����ͬѧ���ּ�ͬѧ��Ƶ�ԭ���װ��Ч�ʲ��ߣ������ڶ�ʱ���ھͻ�˥����Ϊ����������⣬��ԭ�����Ƴ��˴����ŵ�װ�ã���ͼ2��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
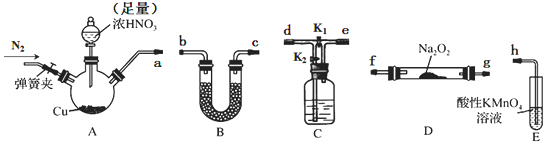
| ���� | ���������� |
| �� | �ر�K2����K1�����ɼ�ͨһ��ʱ��ĵ������н����ɼУ���ʼA�з�Ӧ��һ��ʱ��۲쵽E����Һ��Ϊ����ɫ�� |
| �� | ֹͣA�з�Ӧ�����ɼк�K2���ر�K1������ͨ��N2һ��ʱ�䣮 |
| �� | �����µ�Eװ�ã���ͨһ��ʱ��N2��رյ��ɼУ�ʹA�з�Ӧ�������۲쵽�������벽�������ͬ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����������� | ���� |
| A | ����ҺX�еμ�BaCl2��Һ���а�ɫ�������� | ��ҺX�п��ܺ���SO42- |
| B | ���ʵ���֮��Ϊ2��3��ϡ�����ϡ����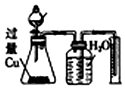 | ��Ӧ��������ƿ����Һ��������CuSO4������ƿ���ռ�����������NO |
| C | ��1mLŨ�Ⱦ�Ϊ0.05mol•L-1NaCl��NaI�Ļ����Һ�еμ�2��0.01mol•L-1 AgNO3��Һ���������ǻ�ɫ | Ksp��AgCl����Ksp��AgI�� |
| D | �����£���pH��ֽ���0.1mol•L-1NaHSO3��Һ��pHԼΪ5 | HSO3-�ĵ���̶ȴ�����ˮ��̶� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����״̬�»���ˮ��Һ������������������ƶ������ӵĻ������ǵ���� | |
| B�� | ������ˮ��Һ����ۻ�״̬�¶����ܵ�������ʽзǵ���� | |
| C�� | �ܵ��������һ���ǵ���� | |
| D�� | ij���������ǵ���ʣ���һ���Ƿǵ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com