
��4�֣��Ķ������������������ϣ�
����һ��
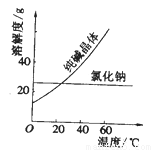
���϶���
|
���� |
�۵�/�� |
�е�/�� |
�ܶ�/g��cm-3 |
�ܽ��� |
|
�Ҷ����� C2H6O2�� |
-11.5 |
198 |
1.11 |
������ˮ���Ҵ� |
|
��������C3H8O3�� |
17.9 |
290 |
1.26 |
�ܸ�ˮ���ƾ�������Ȼ��� |
�ش��������⣨��д��ţ���
A������ B����ȡ�� C�����ܽ⡢�ᾧ�����ˡ��ķ��� D����Һ��
��1�� ��������Ȼ��ƺʹ���Ļ�����з�����������Ӧ��__________��
��2�����Ҷ����ͱ�������������ѷ�����__________��
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��1���Ķ������������������ϣ�����һ��
��1���Ķ������������������ϣ�����һ��| ���� | �۵�/�� | �е�/�� | �ܶ�/g?cm3 | �ܽ��� |
| �Ҷ�����C2H6O2�� | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
| ��������C3H8O3�� | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ��������Ի��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | �۵�/�� | �е�/�� | �ܶ�/g?cm-3 | �ܽ��� |
| �Ҷ����� C2H6O2�� | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
| ��������C3H8O3�� | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ɽ���Ӷ��������л������ķ����֮һ��������һ������Ϊ���й��������ĺ���--�˳��γأ�
��ɽ���Ӷ��������л������ķ����֮һ��������һ������Ϊ���й��������ĺ���--�˳��γأ�| ���� | �۵�/�� | �е�/�� | �ܶ�g?cm-3 | �ܽ��� |
| �Ҷ���C2H6O2 | -11.5 | 198 | 1.11 | ������ˮ ���Ҵ� |
| ������C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���� ��������� ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
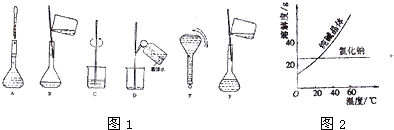
| ���� | �۵�/�� | �е�/�� | �ܶ�/g?cm-3 | �ܽ��� | �Ҷ���C2H6O2 | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� | ������C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���㽭ʡ��һ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
�Ķ������������������ϣ�
����һ 
���϶�
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
| �Ҷ��� C2H6O2 | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
| ������C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com