
̼���������ĵ��ʼ��仯�����ڹ�ũҵ����������������Ҫ�����á�
��1�����̼�Ȼ�ԭ���Ȼ�����ʵ���������Ʊ�������������ص��Ȼ�ѧ����ʽ���£�
2Al2O3(s)��2AlCl3(g)��6C(s)=6AlCl(g)��6CO(g) ��H��a kJ��mol��1
3AlCl(g) ��2Al(l)��AlCl3(g) ��H��b kJ��mol��1
��ӦAl2O3(s)��3C(s)��2Al(l)��3CO(g)�ġ�H= kJ��mol��1���ú�a��b�Ĵ���ʽ��ʾ����
��2���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������ӦC(s)+2NO(g) N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g) ��H="Q" kJ��mol��1��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| ʱ�䣨mol/L�� Ũ�ȣ�mol/L�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
| N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
| CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ʵ�У�ʲô����Ӱ���˻�ѧ��Ӧ���ʣ�
�����ʳƷ��ù�䣬����Ͳ����������� ��
���ۻ�������طų����ݽ��������������������̺ܿ�������� ��
�۹�ҵ�ϳ�������ȼ�Ϸ��飬�����ȼ��Ч�� ��
��ͬ�����ͬŨ�ȵ�������ͬ����С��п����þ����Ӧ����������ǰ����� ��
��ͬ�����ͬŨ�ȵ������������ͬ����С������ȵ�п����Ӧ����������ǰ�߿� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���Ĺ̶�������;��������̵�����Ȼ�̵���ҵ�̵����������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2)������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±�(���ա�N2ѹ��1.0��105 Pa����Ӧʱ��1 h)��
| T/K | 303 | 313 | 323 | 353 |
| NH3������/(10��6 mol) | 4.8 | 5.9 | 6.0 | 2.0 |
 4NH3(g)��3O2(g)��H��a kJ��mol��1
4NH3(g)��3O2(g)��H��a kJ��mol��1 2NH3(g)����H����92 .4 kJ��mol��1
2NH3(g)����H����92 .4 kJ��mol��1 2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�
2NH3(g)����H����92 .4 kJ��mol��1���ֱ��о���T1��T2��T3(T1<T2<T3)�����¶��ºϳɰ����Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��N2����ʼ��ɱ�(��ʼʱN2�����ʵ�����Ϊ1 mol)��N2ƽ��ת���ʵĹ�ϵ����ش�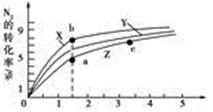
 N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ ��
N2(g)��3H2(g)��ƽ�ⳣ��Ϊ________ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(14��)Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
(1)������ȼ������ʱ������N2��O2�ķ�Ӧ��N2 + O2 2NO���ǵ�������β���к���NO��ԭ��֮һ��
2NO���ǵ�������β���к���NO��ԭ��֮һ��
����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2��g�� + O2��g�� 2NO��g���ġ�H________0(���������)��
2NO��g���ġ�H________0(���������)��
����T3�¶��£���2L�ܱ������г���10molN2��5mo1O2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ�����ʦ�(N2)��___________________�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1 mol����ﵽƽ���N2��ת����Ϊ____________��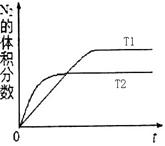
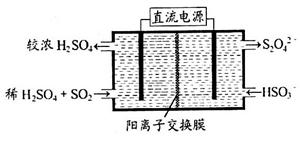
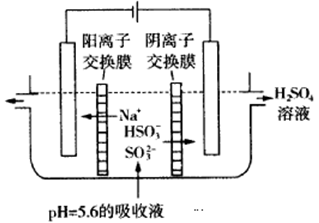
(2)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2���������ų�����Һ������NO2��
�������ĵ缫��ӦʽΪ_____________________��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ��SO32�����ɡ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
(3)һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol/L�Ĵ�����b mol/L
Ba(OH)2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c(Ba2��)��c(CH3COO��)����û����Һ�д���ĵ��볣��Ka��______________________(�ú�a��b�Ĵ���ʽ��ʾ)��
��4������������PM2.5ϸ���Ӱ���(NH4)2SO4��NH4NO3���л������P�ﳾ�ȣ���дһ����SO42����Ϊ�ȵ�����ķ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��֪һ����̼��ˮ�����ķ�ӦΪ��CO(g)��H2O (g) CO2(g)��H2(g) ��800��ʱ�����ݻ�Ϊ2.0L���ܱ������г���2.0mol CO(g)��3.0mol H2O(g)�������¶Ȳ��䣬4 min��Ӧ�ﵽƽ�⣬���CO��ת����Ϊ60%��
CO2(g)��H2(g) ��800��ʱ�����ݻ�Ϊ2.0L���ܱ������г���2.0mol CO(g)��3.0mol H2O(g)�������¶Ȳ��䣬4 min��Ӧ�ﵽƽ�⣬���CO��ת����Ϊ60%��
��1����4 min��H2��ƽ����ѧ��Ӧ���ʡ�
��2������800��ʱ�÷�Ӧ��ƽ�ⳣ����
��3��427��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ9.4�����ϣ�2���еļ������жϸ÷�Ӧ�Ħ�H 0������������������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��Ԫ�صĻ����������������������Ź㷺��Ӧ�á�
��1��400�棬1.01��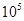 Pa�£��ݻ�Ϊ1��0L���ܱ������г���0.5molSO2, (g)��0.3 molO2 (g)��������Ӧ2SO2(g)��O2(g)
Pa�£��ݻ�Ϊ1��0L���ܱ������г���0.5molSO2, (g)��0.3 molO2 (g)��������Ӧ2SO2(g)��O2(g) 2SO3(g) ��H����198kJ/mol����Ӧ��n(SO3)��n(O2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ
2SO3(g) ��H����198kJ/mol����Ӧ��n(SO3)��n(O2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ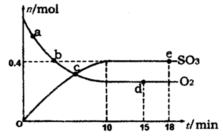 ����Ӧ��ƽ�ⳣ��K��_______��0��10 min����SO2��ʾ��ƽ����Ӧ����_________������ͼ����Ϣ���ж�������������ȷ����_____������ţ���
����Ӧ��ƽ�ⳣ��K��_______��0��10 min����SO2��ʾ��ƽ����Ӧ����_________������ͼ����Ϣ���ж�������������ȷ����_____������ţ���
| A��a��ʱ�̵�����Ӧ���ʱ�b��ʱ�̵Ĵ� |
| B��c��ʱ�̷�Ӧ�ﵽƽ��״̬ |
| C��d���e��ʱ�̵�c(O2)��ͬ |
| D����5 00�棬1.01��105Pa�£���Ӧ�ﵽƽ��ʱ��n( SO3) ��ͼ��e��ʱ�̵�ֵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��֪��Ӧ��CO(g)+H2O(g) H2(g)+CO2(g)
H2(g)+CO2(g)
��427��Cʱ��ƽ�ⳣ����9��4�������Ӧ��ʼʱ��һ����̼��ˮ������Ũ�ȶ���0��01mol��L-1������һ����̼�ڴ˷�Ӧ�����µ�ת���ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ�ܱ������з�����Ӧ�� 2A(g)+B(g) E(g)
E(g)
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ___________ ____����֪�����¶�ʱ��v������>v���棩����ʱKֵ__ __��������С�����䡱�����÷�Ӧ�ġ�H_____0���>������=������<������
��2����1.0mol A��1.0mol B��Ϻ�װ��2L�����з�����Ӧ��E�����ʵ����ı仯��ͼ��ʾ��
��3������E��ƽ����Ӧ����Ϊ________ _________��
������¶��¸÷�Ӧ��ƽ�ⳣ��K= ���������С����1λ����
������ͼ�л���5������A�����ʵ����仯�����ߣ�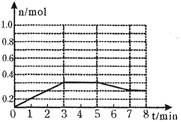
��3����֪����ͼ��ʾ�ı仯�У�ƽ�ⳣ��K���ֲ��䣬����5��7min������E�����ʵ����仯��ԭ�������____________�����ţ���
�ٽ������¶� ���������¶� ��ʹ���˴��� ����������������� ����С����������� ������A�����ʵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯������100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų�������(���)��ʵ���¼����(�ۼ�ֵ)��
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 |
| �������(mL) | 50 | 120 | 232 | 290 | 310 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com