
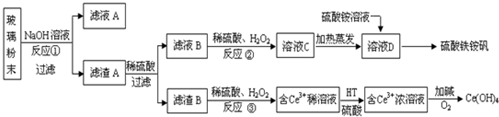
 ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
ijѧ����0.2000mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�| �ζ����� | ���������mL�� | ���ռ������mL�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 2.00 | 24.10 |
| ������ | 20.00 | 4.00 | 24.00 |
���� ��1�����ݼ�ʽ�ζ�����װҺǰӦ����װҺ�������ϴ������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������������V����������Ӱ�죬�Դ��ж�Ũ�ȵ���
��2��������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��3�����ݵζ��ܵĽṹ�;�ȷ�ȶ�����
��4���ȸ������ݵ���Ч�ԣ���ȥ��3�����ݣ�Ȼ�����1��2��ƽ������V��NaOH�������Ÿ���c�����⣩=$\frac{V��������c������}{V�����⣩}$�����㣮
��� �⣺��1�����ݼ�ʽ�ζ�����װҺǰӦ����װҺ�������ϴ��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ�
A�����Ʊ���Һ�Ĺ���NaOH�л���KOH���ʣ����ڵζ�ʱ���ĵ�����ƫ�٣�������������һ��ʱ�����ĵı���Һ�����ƫ����ⶨ���ƫ��A��ȷ��
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ������Һ�����ƫ����ⶨ���ƫ��B��ȷ��
C��ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ����ƿ����������ʵ���ƫ�ζ�ʱ���ĵı���Һ�����ƫ�����Բⶨ���ƫ��C��ȷ��
D���ζ����յ����ʱ�����ֵζ��ܼ��촦����һ����Һ�������ĵı���Һ�����ƫ�����Բⶨ���ƫ��D��ȷ��
�ʴ�Ϊ���٣�ABCD��
��2���ζ�ʱ������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣬���Ե������һ��NaOH��Һʱ����Һ�պ�����ɫ��Ϊ�ۺ죬�Ұ�����ڲ���ɫ��
�ʴ�Ϊ���������һ��NaOH��Һʱ����Һ�պ�����ɫ��Ϊ�ۺ죬�Ұ�����ڲ���ɫ��
��3���ζ����е�Һ�����Ϊ22.60mL��
�ʴ�Ϊ��22.60��
��4�����εζ����ĵ����Ϊ��20.00mL��22.10mL��20.00����ȥ��2�����ݣ�Ȼ�����1��2��ƽ������V��NaOH��=20.00mL��
c�����⣩=$\frac{V��������c������}{V�����⣩}$=$\frac{0.2000mol•{L}^{-1}��20.00mL}{20.00mL}$=0.2000mol•L-1��
�ʴ�Ϊ��0.2000��
���� ���⿼��������к͵ζ�����Ŀ�ѶȲ���עע������к͵ζ���ԭ���������������������ķ�����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͼ��ʾ���ǡ����ܼ��š��еĽ��ܱ�־ | |
| B�� | �ӿ컯ʯȼ�ϵĿ�����ʹ�ã�ֹͣʹ�ú��� | |
| C�� | ��װú̿��������������װ�ã��������귢���� | |
| D�� | �ƹ�ũ�����������μ���������ũҩ��ʹ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��λ�����ڱ��ĵ������ڣ���I A�� | |
| B�� | ������������� | |
| C�� | ���ơ��ء��3�ֵ����У�卑��۵���� | |
| D�� | ����������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Dsԭ�������ڱ���λ�ڵ�7���ڵڢ�B�� | |
| B�� | Ds�ǹ���Ԫ�� | |
| C�� | Dsԭ�ӵĺ��������Ϊ110 | |
| D�� | DsΪ����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ��ⶨ��Ũ�ȣ�
ij�ռ���Һ�к����������ʣ��������ᷴӦ���������к͵ζ��ⶨ��Ũ�ȣ�| ָʾ�� | ʯ�� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0-8.0 | 3.1-4.4 | 8.2-10.0 |
| �ζ���� | ����Һ�����mL�� | �����������Һ�������mL�� | ||
| �ζ�ǰ | �ζ��� | ���ĵ���� | ||
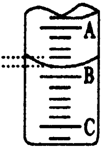
| 1 | 20 | 0.50 | 25.12 | 24.62 |
| 2 | 20 | ��ͼ | ��ͼ | ��д24.60 |
| 3 | 20 | 6.00 | 30.58 | 24.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ��֮���γɹ��ۼ�ʱ�����γ�2���Ҽ� | |
| B�� | ������Ʒ�����ߣ�������û�б��ƻ� | |
| C�� | �¶�Խ�ߣ������ĵ�����Խ�� | |
| D�� | �κξ��嶼����ѧ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com