
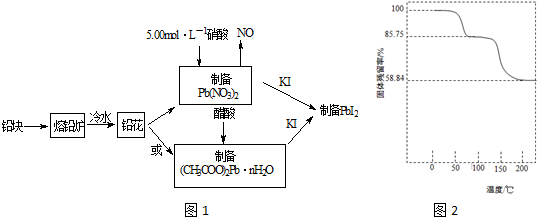
���� �÷�Ǧ��Ϊԭ�Ϻϳ�PbI2�����̣�Ϊ���������ᷴӦ�ĽӴ�������ӿ��ܽⷴӦ���ʣ���Ǧ���Ƴ�Ǧ����;��һ��3Pb+8HNO3=3Pb��NO3��2+2NO��+4H2O��Pb��NO3��2+KI=PbI2��+KNO3��;������Pb��NO3��2+2CH3COOH+nH2O=��CH3COO��2Pb•nH2O+2HNO3����CH3COO��2Pb•nH2O+2KI=PbI2��+2CH3COOK+nH2O��
��1��Ǧ���Ƴ�Ǧ��Ϊ����������������Ӱ�췴Ӧ���ʵ�����ȥ������
��2��Ǧ�ܽ���ϡ����ķ�Ӧ����ʽΪ3Pb+8HNO3=3Pb��NO3��2+2NO+4H2O�������ܽ��Ǧ�����ʵ����ɼ������ĵ�������Һ�������
��3���ɼ�����ʼ��������Ϊ100g������ȵ�75��ʱ��ȫʧȥ�ᾧˮ���������ǹ������������������ˮ�����ʵ���������Ǧ�����ʵ������������ߵ����ʵ���֮�ȿ�ȷ��n��ֵ��
��4����������к͵�ԭ���ɼ������Һ�е�H+��Ũ�ȣ�����Ϲ�ϵʽȷ����Һ��Pb2+��Ũ�ȣ��ټ���Ksp
��� �⣺��1����Ǧ���Ƴ�Ǧ������Ϊ���������ᷴӦ�ĽӴ�������ӿ��ܽⷴӦ���ʣ��ʴ�Ϊ����������ĽӴ��棬�ӿ��ܽⷴӦ���ʣ�
��2��31.05gǦ�����ʵ���Ϊ$\frac{31.05g}{207g/mol}$=0.15mol�����ݷ�Ӧ����ʽ��֪���ĵ�HNO3�����ʵ���Ϊ0.15mol��$\frac{8}{3}$=0.4mol����������Һ�����Ϊ0.4mol��5.00mol•L-1=0.08L=80.0mL���ʴ�Ϊ��80.0��
��3��������Ʒ����ʼ����Ϊ100�ˣ����ݹ�������ʵĹ�ʽ��֪��75��ʱʣ�����Ϊ87.75�ˣ����ɵ�ˮ����Ϊ100g-87.75g=12.25g�������Ǧ��ˮ�����ʵ���֮��Ϊ$\frac{85.75g}{325g/mol}$��$\frac{12.25g}{18g/mol}$=1��3����n=3���ʴ�Ϊ��3��
��4��n��H+��=n��NaOH��=0.002500 mol•L-1��20.00mL��10-3L•mL-1=5.000��10-5mol
n[Pb2+��aq��]=$\frac{1}{2}$n��H+��=2.500��10-5mol
c��Pb2+��=$\frac{2.500��10{\;}^{-5}mol}{25.00mL��10{\;}^{-3}L/mL}$=1.000��10-3 mol•L-1
Ksp��PbI2��=c��Pb2+��•c2��I-��=4c3��Pb2+��=4����1.000��10-3��3=4.000��10-9��
������ʱPbI2 ��Ksp Ϊ��4.000��10-9��
���� �������Ʊ��⻯ǦΪ���壬�ص㿼��ѧ�������ݴ����������ѶȽϴ�ѧ���ķ������⣬������������Ҫ��ϸߣ������ʵ�ѵ��������Ŀ��


 ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
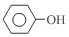 +3Br2��
+3Br2��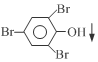 +3HBr
+3HBr�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
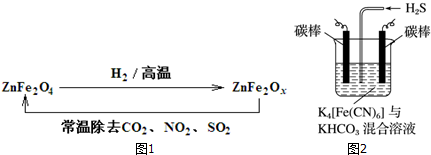
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
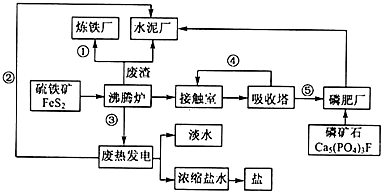 ��̬��ҵ���Ľ��裬�����������ֻ��������Ҫ����ѭ���������ۺͳ�ֿ��Ǿ��õĿɳ�����չ����ͼ��ij��ҵ��Ƶ�����-���-ˮ����������ˮ-��ˮ���ã���-��-��������������̬��ҵ������ͼ��
��̬��ҵ���Ľ��裬�����������ֻ��������Ҫ����ѭ���������ۺͳ�ֿ��Ǿ��õĿɳ�����չ����ͼ��ij��ҵ��Ƶ�����-���-ˮ����������ˮ-��ˮ���ã���-��-��������������̬��ҵ������ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
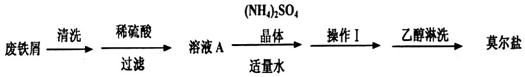
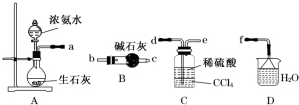
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ������ | �ܶȣ�g•cm-3�� | ˮ���� | �е㣨�棩 |
| ������ | 1.05 | ���� | 118.1 |
| ������ | 0.80 | �� | 117.2 |
| ������ | 0.77 | ���� | 142.0 |
| ���������� | 0.90 | �� | 126.5 |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��KNO3�Ĵ��� | 1 | 2 | 3 | 4 |
| ��KNO3��������g�� | 15 | 30 | 45 | 60 |
| ������Һ������g�� | 115 | 130 | 145 | 145 |
| A�� | ���¶���KNO3���ܽ��Ϊ45g | |
| B�� | ��2�μ���KNO3��������ҺΪ��������Һ | |
| C�� | KNO3�������ܵ��� | |
| D�� | ��4�μ���KNO3��������Һ����������Ϊ45% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ʵ�������λ | B�� | �������ʵ�������λ | ||
| C�� | �������ʵ����ĵ�λ | D�� | �����������������Ӹ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com