
[2012”¤±±¾©³ÆŃōŅ»Ä£]£Ø12·Ö£©øßĢśĖįÄĘ£ØNa2FeO4£©¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬ŹĒŅ»ÖÖŠĀŠĶµÄĀĢÉ«¾»Ė®Ļū¶¾¼Į”£¹¤ŅµÉĻæÉŅŌĶعż“ĪĀČĖįÄĘŃõ»Æ·ØÖʱøøßĢśĖįÄĘ£¬Éś²ś¹ż³ĢČēĻĀ£ŗ

»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¾¹ż²½Öč¢Łŗ󣬼ÓČėNaOH¹ĢĢåµÄŌŅņŹĒ____ ____”£
£Ø2£©²½Öč¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_____ ___”£
£Ø3£©“ÓČÜŅŗ¢ńÖŠ·ÖĄė³öNa2FeO4ŗ󣬻¹ÓŠø±²śĘ·Na2SO4 ”¢NaCl£¬Ōņ²½Öč¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___ _____”£
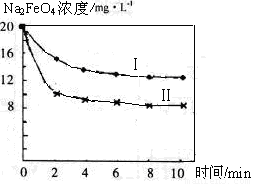
£Ø4£©½«Ņ»¶ØĮæµÄNa2FeO4Ķ¶Čėµ½pH²»Ķ¬µÄĪŪĖ®ÖŠ£ØĪŪĖ®ÖŠĘäÓą³É·Ö¾łĻąĶ¬£©£¬ČÜŅŗÖŠNa2FeO4ÅØ¶Č±ä»ÆČēĻĀĶ¼ĒśĻߢń”¢¢ņĖłŹ¾£¬ŹŌĶĘ²āĒśĻßI±ČĒśĻßII¶ŌÓ¦µÄĪŪĖ®pH_____£ØĢī”°øß”±»ņ”°µĶ”±£©”£
£Ø5£©Ķعż¼ĘĖćµĆÖŖNa2FeO4µÄĻū¶¾Š§ĀŹ£ØŅŌµ„Ī»ÖŹĮæµĆµ½µÄµē×ÓŹż±ķŹ¾£©±ČĀČĘųµÄ_____£ØĢī”°øß”±»ņ”°µĶ”±£©£¬ÓĆøßĢśĖįÄĘ“śĢęĀČĘų×÷¾»Ė®Ļū¶¾¼ĮµÄÓŵćŹĒ____ _
£Ø“š³öĮ½µć¼“æÉ£©”£
£Ø12·Ö£©£Ø1£©Na2FeO4Ö»ŌŚ¼īŠŌ»·¾³ÖŠĪČ¶Ø“ęŌŚ£¬ĖłŅŌ¼ÓČėĒāŃõ»ÆÄĘæÉŅŌµ÷½ŚČÜŅŗĻŌ¼īŠŌ
£Ø2£©2Fe2+£«H2O2£«2H+£½2Fe3+£«2H2O
£Ø3£©2Fe3+£«3ClO££«10OH££½2FeO42££«3Cl££«5H2O
£Ø4£©øß
£Ø5£©µĶ ¼ČÄÜĻū¶¾É±¾śÓÖÄܾ»Ė®£Ø»ņĪŽ¶¾»ņ·½±ć±£“ęµČ£©
”¾½āĪö”æ£Ø1£©øł¾ŻæņĶ¼ÖŠµÄĢįŹ¾æÉÖŖ£¬Na2FeO4Ö»ŌŚ¼īŠŌ»·¾³ÖŠĪČ¶Ø“ęŌŚ£¬ĖłŅŌ¼ÓČėĒāŃõ»ÆÄĘæÉŅŌµ÷½ŚČÜŅŗĻŌ¼īŠŌ”£
£Ø2£©ŌŚĖįŠŌĢõ¼žĻĀ£¬H2O2½«Fe2+Ńõ»Æ³ÉFe3+£¬×ŌÉķ±»»¹ŌÉś³ÉH2O£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe2+£«H2O2£«2H+£½2Fe3+£«2H2O”£
£Ø3£©²½Öč¢ŪÖŠ²Ī¼Ó·“Ó¦µÄĄė×ÓÓŠFe3+”¢ClO£ŗĶOH££¬³żÉś³ÉFeO42£ŗĶCl£Ķā»¹ÓŠH2OÉś³É£¬¹ŹøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe3+£«3ClO££«10OH££½2FeO42££«3Cl££«5H2O”£
£Ø4£©Na2FeO4ŌŚ¼īŠŌ»·¾³ÖŠĪČ¶Ø“ęŌŚ£¬ĪŪĖ®µÄpHŌ½øߣ¬Na2FeO4µÄÅضČŌ½“󣬹ŹĒśĻßI±ČĒśĻßII¶ŌÓ¦µÄĪŪĖ®pHøß”£
£Ø5£©1gNa2FeO4ÄܵƵ½µē×ÓµÄĪļÖŹµÄĮæĪŖ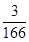 mol”Ö0.018mol£¬1gCl2ÄܵƵ½µē×ÓµÄĪļÖŹµÄĮæĪŖ
mol”Ö0.018mol£¬1gCl2ÄܵƵ½µē×ÓµÄĪļÖŹµÄĮæĪŖ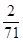 mol”Ö0.028mol£¬¹ŹNa2FeO4µÄĻū¶¾Š§ĀŹ±ČĀČĘųµÄµĶ”£
mol”Ö0.028mol£¬¹ŹNa2FeO4µÄĻū¶¾Š§ĀŹ±ČĀČĘųµÄµĶ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŠĀæĪ±źøßČżÅäĢ×ŌĀæ¼£ØŅ»£©»ÆѧŹŌ¾ķ£ØB¾ķ£©£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
[2012”¤±±¾©³ÆŃōŅ»Ä£]£Ø12·Ö£©øßĢśĖįÄĘ£ØNa2FeO4£©¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬ŹĒŅ»ÖÖŠĀŠĶµÄĀĢÉ«¾»Ė®Ļū¶¾¼Į”£¹¤ŅµÉĻæÉŅŌĶعż“ĪĀČĖįÄĘŃõ»Æ·ØÖʱøøßĢśĖįÄĘ£¬Éś²ś¹ż³ĢČēĻĀ£ŗ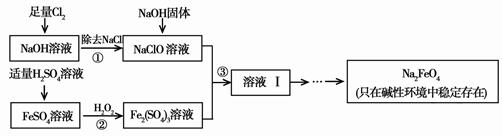
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¾¹ż²½Öč¢Łŗ󣬼ÓČėNaOH¹ĢĢåµÄŌŅņŹĒ____ ____”£
£Ø2£©²½Öč¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_____ ___”£
£Ø3£©“ÓČÜŅŗ¢ńÖŠ·ÖĄė³öNa2FeO4ŗ󣬻¹ÓŠø±²śĘ·Na2SO4”¢NaCl£¬Ōņ²½Öč¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___ _____”£
£Ø4£©½«Ņ»¶ØĮæµÄNa2FeO4Ķ¶Čėµ½pH²»Ķ¬µÄĪŪĖ®ÖŠ£ØĪŪĖ®ÖŠĘäÓą³É·Ö¾łĻąĶ¬£©£¬ČÜŅŗÖŠNa2FeO4ÅØ¶Č±ä»ÆČēĻĀĶ¼ĒśĻߢń”¢¢ņĖłŹ¾£¬ŹŌĶĘ²āĒśĻßI±ČĒśĻßII¶ŌÓ¦µÄĪŪĖ®pH_____£ØĢī”°øß”±»ņ”°µĶ”±£©”£
£Ø5£©Ķعż¼ĘĖćµĆÖŖNa2FeO4µÄĻū¶¾Š§ĀŹ£ØŅŌµ„Ī»ÖŹĮæµĆµ½µÄµē×ÓŹż±ķŹ¾£©±ČĀČĘųµÄ_____£ØĢī”°øß”±»ņ”°µĶ”±£©£¬ÓĆøßĢśĖįÄĘ“śĢęĀČĘų×÷¾»Ė®Ļū¶¾¼ĮµÄÓŵćŹĒ____ _
£Ø“š³öĮ½µć¼“æÉ£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com