
| Ԫ�� | Al | B | Be | C | Cl | F | Li |
| X����ֵ | 1.5 | 2.0 | 1.5 | 2.5 | 2.8 | 4.0 | 1.0 |
| Ԫ�� | Mg | Na | O | P | S | Si | |
| X����ֵ | 1.2 | 0.9 | 3.5 | 2.1 | 2.5 | 1.7 | |
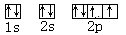 ��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף�
��5��+7��HClO4 ��6��ԭ�ӹ��ף����ף� ��
��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1s<2s<3s | B��2p<3p<4p | C��3s<3p<3d | D��4s>3d>3p |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����зǽ���Ԫ�ض��ֲ���p�� |
| B������������Ϊ2��Ԫ�ض��ֲ���s�� |
| C��Ԫ�����ڱ��Т�B�嵽��B��10�����е�Ԫ�ض��ǽ���Ԫ�� |
| D��ͬһ����Ԫ�ش��ϵ��£������Գ������Ա仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
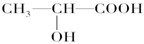
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | A | B | C | D |
| ԭ�Ӱ뾶(nm) | 0.130 | 0.118 | 0.090 | 0.102 |
| ��Ҫ���ϼ� | ��2 | ��3 | ��2 | ��6����2 |
| Ԫ�� | E | F | G | H |
| ԭ�Ӱ뾶(nm) | 0.073 | 0.154 | 0.037 | 0.099 |
| ��Ҫ���ϼ� | ��2 | ��1 | ��1 | ��7����1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�
R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�ء�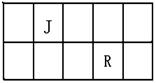
 ��
�� ��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __��
��ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ___ __���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
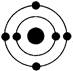

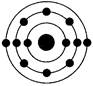
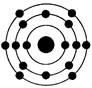
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
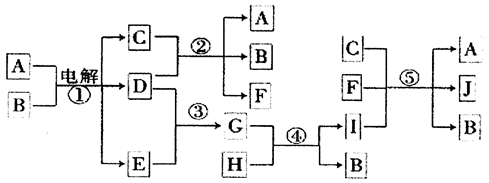
 __________________��
__________________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com