
ĻÖĻņŗ¬6 mol KIµÄĮņĖįČÜŅŗÖŠÖšµĪ¼ÓČėKBrO3ČÜŅŗ£¬Õūøö¹ż³ĢÖŠŗ¬µāĪļÖŹµÄĪļÖŹµÄĮæÓėĖł¼ÓČėKBrO3µÄĪļÖŹµÄĮæµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ŅŃÖŖ
¢ŁBrO3£+6IŅ»+6H+=3I2+Br£+3H2O£»
¢Ś2BrO3£ +I2 = 2IO3£ + Br2£»
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©bµćŹ±£¬KI·“Ó¦ĶźČ«£¬ŌņĻūŗĵÄŃõ»Æ¼ĮÓė»¹Ō¼ĮĪļÖŹµÄĮæÖ®±ČĪŖ £¬»¹Ō²śĪļŹĒ ”£
£Ø2£©b”śc¹ż³ĢÖŠÖ»ÓŠŅ»ÖÖŌŖĖŲµÄ»ÆŗĻ¼Ū·¢Éś±ä»Æ£¬Š“³öøĆ¹ż³ĢµÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø3£©ÓÉ·“Ó¦¢ŚÓŠĶ¬Ń§ÓÉ“ĖµĆ³öŃõ»ÆŠŌ£ŗI2£¾Br2µÄ½įĀŪ£¬ÄćČĻĪŖŹĒ·ńÕżČ·£¬²¢ĖµĆ÷ĄķÓÉ
ӣ
£Ø4£©ŗ¬6 mol KIµÄĮņĖįČÜŅŗĖłÄÜĻūŗÄn(KBrO3)µÄ×ī“óÖµ
ĪŖ ”£
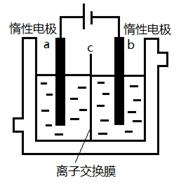
£Ø5£©¼ÓµāŹ³ŃĪÖŠŗ¬ÓŠµāĖį¼Ų(KIO3)£¬ĻÖŅŌµē½ā·ØÖʱøµāĖį¼Ų£¬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾”£ĻČ½«Ņ»¶ØĮæµÄµāČÜÓŚ¹żĮæĒāŃõ»Æ¼ŲČÜŅŗ£¬·¢Éś·“Ó¦£ŗ3I2+6KOH=5KI+KIO3+3H2O£¬½«øĆČÜŅŗ¼ÓČėŃō¼«Ēų£¬Įķ½«ĒāŃõ»Æ¼ŲČÜŅŗ¼ÓČėŅõ¼«Ēų£¬æŖŹ¼µē½ā”£Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ £»Ņõ¼«¹Ū²ģµ½µÄĻÖĻóŹĒ £»

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø16·Ö£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢G”¢H”¢I¾łĪŖĘųĢ壬JĪŖ³£¼ūµÄŅŗĢ¬ĪļÖŹ£¬A”¢B”¢C”¢I”¢MĪŖµ„ÖŹ£¬ĒŅMĪŖ³£ÓĆ½šŹō£¬GŗĶHĻąÓöŹ±²śÉś°×ŃĢ£¬ĖüĆĒ“ęŌŚČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņ²śĪļŅŃŹ”ĀŌ£©£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©A·Ö×ӵĵē×ÓŹ½ŹĒ £¬G·Ö×ÓµÄæռ乹ŠĶĪŖ ”£
£Ø2£©³£ĪĀĻĀ£¬pHÖµ¾łĪŖ5µÄHČÜŅŗŗĶKČÜŅŗÖŠÓÉĖ®µēĄėµÄc(H+)Ö®±ČĪŖ ”£
£Ø3£©ČōĻņXµÄĖ®ČÜŅŗÖŠĶØČėG£¬²śÉśµÄĻÖĻóŹĒ £¬NÓėXÖŠ¶¼ŗ¬MµÄŌŖĖŲ£¬Ęä»ÆŗĻ¼ŪŹĒ·ńĻąĶ¬ ”£
£Ø4£©Š“³öX+C”śYµÄĄė×Ó·½³ĢŹ½ ”£
MÓėĘųĢ¬JŌŚøßĪĀŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø5£©ĶĮČĄ½ŗĮ£“ųøŗµē£¬ŗ¬ĻąĶ¬ÖŹĮæAŌŖĖŲµÄZŗĶKŹ©ÓƵ½µ¾Ģļŗ󣬊§¹ūøüŗƵďĒ - £ØĢī»ÆѧŹ½£©”£
£Ø6£©ŌŚĶس£×“æöĻĀ£¬Čō1 g BĘųĢåŌŚCĘųĢåÖŠČ¼ÉÕÉś³ÉHĘųĢåŹ±·Å³ö92.3 kJČČĮ棬Ōņ2 mol HĘųĢåĶźČ«·Ö½āÉś³ÉCĘųĢåŗĶBĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģĖÄ“ØŹ”³É¶¼Ķā¹śÓļѧŠ£øßČż2ŌĀŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢G”¢H”¢I¾łĪŖĘųĢ壬JĪŖ³£¼ūµÄŅŗĢ¬ĪļÖŹ£¬A”¢B”¢C”¢I”¢MĪŖµ„ÖŹ£¬ĒŅMĪŖ³£ÓĆ½šŹō£¬GŗĶHĻąÓöŹ±²śÉś°×ŃĢ£¬ĖüĆĒ“ęŌŚČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņ²śĪļŅŃŹ”ĀŌ£©£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©A·Ö×ӵĵē×ÓŹ½ŹĒ £¬G·Ö×ÓµÄæռ乹ŠĶĪŖ ”£
£Ø2£©³£ĪĀĻĀ£¬pHÖµ¾łĪŖ5µÄHČÜŅŗŗĶKČÜŅŗÖŠÓÉĖ®µēĄėµÄc(H+)Ö®±ČĪŖ ”£
£Ø3£©ČōĻņXµÄĖ®ČÜŅŗÖŠĶØČėG£¬²śÉśµÄĻÖĻóŹĒ £¬NÓėXÖŠ¶¼ŗ¬MµÄŌŖĖŲ£¬Ęä»ÆŗĻ¼ŪŹĒ·ńĻąĶ¬ ”£
£Ø4£©Š“³öX+C”śYµÄĄė×Ó·½³ĢŹ½ ”£
MÓėĘųĢ¬JŌŚøßĪĀŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø5£©ĶĮČĄ½ŗĮ£“ųøŗµē£¬ŗ¬ĻąĶ¬ÖŹĮæAŌŖĖŲµÄZŗĶKŹ©ÓƵ½µ¾Ģļŗ󣬊§¹ūøüŗƵďĒ - £ØĢī»ÆѧŹ½£©”£
£Ø6£©ŌŚĶس£×“æöĻĀ£¬Čō1 g BĘųĢåŌŚCĘųĢåÖŠČ¼ÉÕÉś³ÉHĘųĢåŹ±·Å³ö92.3 kJČČĮ棬Ōņ2 mol HĘųĢåĶźČ«·Ö½āÉś³ÉCĘųĢåŗĶBĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012Ń§ÄźÉ½¶«Ź”¼ĆÄžŹŠøßČżÉĻѧʌʌĩ½×¶ĪŠŌ½Ģѧ֏Įæ¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕ
£Ø13·Ö£©ŌĖÓĆ»Æѧ·“Ó¦ŌĄķŃŠ¾æµŖ”¢Įņ”¢ĀČ”¢µāµČµ„ÖŹ¼°Ęä»ÆŗĻĪļµÄ·“Ó¦ÓŠÖŲŅŖŅāŅ唣
£Ø1£©ĮņĖįÉś²śÖŠ£¬SO2“ß»ÆŃõ»ÆÉś³ÉSO3£ŗ
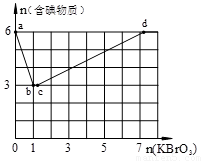
 »ģŗĻĢåĻµÖŠSO3µÄ°Ł·Öŗ¬ĮæŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĒśĻßÉĻČĪŗĪŅ»µć¶¼±ķŹ¾Ę½ŗāדĢ¬£©”£øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ
»ģŗĻĢåĻµÖŠSO3µÄ°Ł·Öŗ¬ĮæŗĶĪĀ¶ČµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĒśĻßÉĻČĪŗĪŅ»µć¶¼±ķŹ¾Ę½ŗāדĢ¬£©”£øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗ

¢Ł µÄ”÷H””””””””0£ØĢī”°>”±
µÄ”÷H””””””””0£ØĢī”°>”±
»ņ”°<”±£©£»ČōŌŚŗćĪĀ”¢ŗćŃ¹Ģõ¼žĻĀĻņÉĻŹöĘ½ŗāĢåĻµÖŠĶØČėŗ¤Ęų£¬
Ę½ŗā””””””””””ŅĘ¶Æ£ØĢī”°Ļņ×ó”±”¢”°ĻņÓŅ”±»ņ”°²»”±£©£»
¢ŚČōĪĀ¶ČĪŖT1”¢T2£¬·“Ó¦µÄĘ½ŗā³£Źż·Ö±šĪŖK1”¢K2£¬
ŌņK1 K2£»·“Ó¦½ųŠŠµ½×“ Ģ¬DŹ±£¬VÕż vÄę£ØĢī”°>”±”¢”°<”±»ņ”°=£©
£Ø2£©µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©Ņµ
Éś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ”£
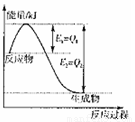
¢Ł Ķ¼ŹĒŅ»¶ØµÄĪĀ¶ČŗĶŃ¹ĒæĻĀŹĒN2ŗĶH2·“Ӧɜ³É1molNH3¹ż
³ĢÖŠÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĒėŠ“³ö¹¤ŅµŗĻ³É°±µÄČČ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
”””””””””””””””££Ø”÷HµÄŹżÖµÓĆŗ¬×ÖÄøQ1”¢Q2µÄ“śŹżŹ½±ķŹ¾£©
¢Ś°±ĘųČÜÓŚĖ®µĆµ½°±Ė®”£ŌŚ25”ćCĻĀ£¬½«a mol”¤L£1µÄ°±Ė®Óė
b mol”¤L£1µÄŃĪĖįµČĢå»ż»ģŗĻ£¬·“Ó¦ŗóČÜŅŗÖŠĻŌÖŠŠŌ£¬Ōņ
c£ØNH+4£©”” c£ØCl££©£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©£»ÓĆŗ¬aŗĶbµÄ“śŹżŹ½±ķŹ¾øĆ»ģŗĻČÜŅŗÖŠŅ»Ė®ŗĻ³ö°±µÄµēĄėĘ½ŗā³£Źż±ķ“ļŹ½”””””””””””””””””””””” ”£
£Ø3£©ŗ£Ė®ÖŠŗ¬ÓŠ“óĮæµÄŌŖĖŲ£¬³£ĮæŌŖĖŲČēĀČ”¢Ī¢ĮæŌŖĖŲČēµāŌŚŗ£Ė®ÖŠ¾łŅŌ»ÆŗĻĢ¬“ęŌŚ”£ŅŃÖŖ£ŗ25”ꏱ£¬KSP(AgCl)=1.6”Į10-10mol2”¤L-2”¢KSP£ØAgI£©=1.5”Į10-16mol”¤L-2£¬ŌŚ25”ęĻĀ£¬Ļņ100mL 0.002mol”¤L-1µÄNaClČÜŅŗÖŠÖšµĪ¼ÓČė100mL 0.002mol”¤L-1ĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É”£“Ó³ĮµķČܽāĘ½ŗāµÄ½Ē¶Č½āŹĶ²śÉś³ĮµķµÄŌŅņŹĒ””””””””””””””””””””£¬Ļņ·“Ó¦ŗóµÄ×ĒŅŗÖŠ£¬¼ĢŠų¼ÓČė0.1mol”¤L£1µÄNaIČÜŅŗ£¬æ“µ½µÄĻÖĻóŹĒ””””””””””””””””””””””£¬²śÉśøĆĻÖĻóµÄŌŅņŹĒ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”””””””””””””””””””””””””””””””””””””””””””””””””””” ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğĖÄ“ØŹ”øßČż2ŌĀŌĀæ¼£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©ŅŃÖŖA”¢B”¢C”¢D”¢E”¢G”¢H”¢I¾łĪŖĘųĢ壬JĪŖ³£¼ūµÄŅŗĢ¬ĪļÖŹ£¬A”¢B”¢C”¢I”¢MĪŖµ„ÖŹ£¬ĒŅMĪŖ³£ÓĆ½šŹō£¬GŗĶHĻąÓöŹ±²śÉś°×ŃĢ£¬ĖüĆĒ“ęŌŚČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£ØĶ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņ²śĪļŅŃŹ”ĀŌ£©£¬Ēė»Ų“šÓŠ¹ŲĪŹĢā£ŗ

£Ø1£©A·Ö×ӵĵē×ÓŹ½ŹĒ £¬G·Ö×ÓµÄæռ乹ŠĶĪŖ ”£
£Ø2£©³£ĪĀĻĀ£¬pHÖµ¾łĪŖ5µÄHČÜŅŗŗĶKČÜŅŗÖŠÓÉĖ®µēĄėµÄc(H+)Ö®±ČĪŖ ”£
£Ø3£©ČōĻņXµÄĖ®ČÜŅŗÖŠĶØČėG£¬²śÉśµÄĻÖĻóŹĒ £¬NÓėXÖŠ¶¼ŗ¬MµÄŌŖĖŲ£¬Ęä»ÆŗĻ¼ŪŹĒ·ńĻąĶ¬ ”£
£Ø4£©Š“³öX+C”śYµÄĄė×Ó·½³ĢŹ½ ”£
MÓėĘųĢ¬JŌŚøßĪĀŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø5£©ĶĮČĄ½ŗĮ£“ųøŗµē£¬ŗ¬ĻąĶ¬ÖŹĮæAŌŖĖŲµÄZŗĶKŹ©ÓƵ½µ¾Ģļŗ󣬊§¹ūøüŗƵďĒ - £ØĢī»ÆѧŹ½£©”£
£Ø6£©ŌŚĶس£×“æöĻĀ£¬Čō1 g BĘųĢåŌŚCĘųĢåÖŠČ¼ÉÕÉś³ÉHĘųĢåŹ±·Å³ö92.3 kJČČĮ棬Ōņ2 mol HĘųĢåĶźČ«·Ö½āÉś³ÉCĘųĢåŗĶBĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ĻņµŚŅ»·Ż2.00 mLČÜŅŗ¼ÓČėAgNO3ČÜŅŗÓŠ³ĮµķÉś³É
(2)ĻņµŚ¶ž·Ż100 mLČÜŅŗ¼Ó×ćĮæNaOHČÜŅŗŗó¼ÓČČ£¬ŹÕ¼Æµ½ĘųĢå0.04 mol(ÉčĘųĢåČ«²æŅŻ³ö)
(3)ĻņµŚČż·Ż100 mLČÜŅŗ¼Ó×ćĮæBaCl2ČÜŅŗŗ󣬵ĆøÉŌļ³Įµķ
A.K+Ņ»¶Ø“ęŌŚ B.100 mLČÜŅŗÖŠŗ¬0.01 mol ![]()
C.Cl-²»æÉÄÜ“ęŌŚ D.Ba2+Ņ»¶Ø²»“ęŌŚ£¬Mg2+æÉÄÜ“ęŌŚ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com