
£Ø16·Ö£©¼×“¼ŹĒŠĀŠĶµÄĘū³µ¶ÆĮ¦Č¼ĮĻ”£¹¤ŅµÉĻæÉĶعżH2ŗĶCO»ÆŗĻÖʱø¼×“¼£¬øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ2H2(g)£«CO(g) CH3OH(g)
CH3OH(g) 
£Ø1£©ŅŃÖŖ£ŗ



1 mol¼×“¼ĘųĢåĶźČ«Č¼ÉÕÉś³ÉCO ŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
ŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ĻĀĮŠ“ėŹ©ÖŠÓŠĄūÓŚĢįøß2H2(g)£«CO(g) CH3OH(g)·“Ó¦ĖŁĀŹµÄŹĒ £ØĖ«Ń”)”£
CH3OH(g)·“Ó¦ĖŁĀŹµÄŹĒ £ØĖ«Ń”)”£
| A£®·ÖĄė³öCH3OH | B£®ÉżøßĪĀ¶Č | C£®¼õŠ”Ń¹Ēæ | D£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ |

 CH3OH(g)µÄĘ½ŗā³£Źż”££ØŠ“³ö¼ĘĖć¹ż³Ģ£©
CH3OH(g)µÄĘ½ŗā³£Źż”££ØŠ“³ö¼ĘĖć¹ż³Ģ£© (1) 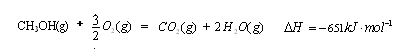 (3·Ö)
(3·Ö)
£Ø2£©BD £Ø4·Ö£© £Ø3£©¢Ł230”ę£Ø3·Ö£©
¢ŚĘäĖūĢõ¼ž²»±ä£¬COµÄĘ½ŗā×Ŗ»ÆĀŹĖę×ÅH2ÓėCOµÄĘšŹ¼×é³É±ČŌö“ó¶ųŌö“ó£Ø»ņ£ŗĘäĖūĢõ¼ž²»±ä£¬COµÄĘ½ŗā×Ŗ»ÆĀŹĖęĪĀ¶ČÉżø߶ų½µµĶ”££© £Ø2·Ö£©
£Ø4£©aµćH2ÓėCOµÄĘšŹ¼×é³É±ČĪŖ1.5£¬COµÄĘ½ŗā×Ŗ»ÆĀŹĪŖ50£„”££Ø1·Ö£©
2H2(g)£«CO(g) CH3OH(g)
CH3OH(g)
ĘšŹ¼ĪļÖŹµÄĮæ(mol) 1.5 1 0
×Ŗ»ÆĪļÖŹµÄĮæ(mol£© 1 0.5 0.5
Ę½ŗāĪļÖŹµÄĮæ(mol) 0.5 0.5 0.5
Ę½ŗāÅضČ(mol/L) 0.5 0.5 0.5 £Ø1·Ö£©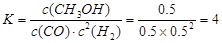 £Ø2·Ö£©
£Ø2·Ö£©
½āĪö


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ³£ĪĀ |
| 1 |
| 2 |
| 1 |
| 2 |
| »Æѧ¼ü | C-C | C-H | H-H | C-O | C”ŌO | H-O |
| ¼üÄÜ/kJ?mol-1 | 348 | 413 | 436 | 358 | 1072 | 463 |
| 3 |
| 2 |
| 3 |
| 2 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| »Æѧ¼ü | C-C | C-H | H-H | C-O | C”ŌO£ØCO£© | H-O |
| ¼üÄÜ/kJ?mol-1 | 348 | 413 | 436 | 358 | 1072 | 463 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”Į¬ÖŻŹŠøßČż10ŌĀŌĀæ¼ĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø16·Ö£©¼×“¼ŹĒŠĀŠĶµÄĘū³µ¶ÆĮ¦Č¼ĮĻ”£¹¤ŅµÉĻæÉĶعżH2ŗĶCO»ÆŗĻÖʱø¼×“¼£¬øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ2H2(g)£«CO(g) CH3OH(g)
CH3OH(g)
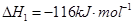
£Ø1£©ŅŃÖŖ£ŗ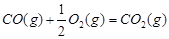
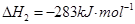
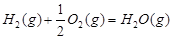
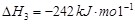
1
mol¼×“¼ĘųĢåĶźČ«Č¼ÉÕÉś³ÉCO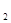 ŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
ŗĶĖ®ÕōĘųµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
£Ø2£©ĻĀĮŠ“ėŹ©ÖŠÓŠĄūÓŚĢįøß2H2(g)£«CO(g) CH3OH(g)·“Ó¦ĖŁĀŹµÄŹĒ
£ØĖ«Ń”)”£
CH3OH(g)·“Ó¦ĖŁĀŹµÄŹĒ
£ØĖ«Ń”)”£
A£®·ÖĄė³öCH3OH B£®ÉżøßĪĀ¶Č C£®¼õŠ”Ń¹Ēæ D£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ
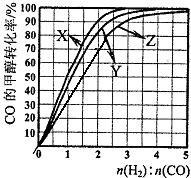
£Ø3£©£Ø3£©ÓĆH2ŗĶCO»ÆŗĻÖʱø¼×“¼µÄ·“Ó¦ÖŠ£¬Čō·“Ó¦µÄČŻ»żĪŖ1LµÄŗćČŻČŻĘ÷£¬·Ö±šŌŚ230”ę”¢250”ęŗĶ270”ęĻĀ£¬øıäH2ŗĶCOµÄĘšŹ¼×é³É±Č£ØĘšŹ¼Ź±COµÄĪļÖŹµÄĮæ¹Ģ¶ØĪŖ1mol£©½ųŠŠŹµŃ飬½į¹ūČēĻĀĶ¼ĖłŹ¾£ØĶ¼ÖŠĒśĻßÉĻµÄµć¶¼ĪŖŅ»¶ØĪĀ¶ČĻĀ”¢Ņ»¶Ø×é³ÉĻĀµÄĘ½ŗāµć£©£ŗ
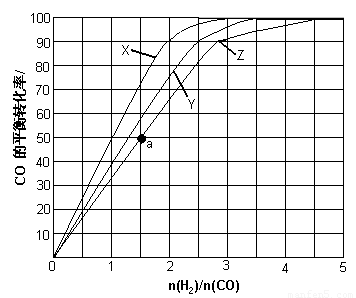
¢ŁĒśĻßX¶ŌÓ¦µÄĪĀ¶ČŹĒ ”£
¢Ś“ÓĶ¼ÖŠæÉŅŌµĆ³öµÄ½įĀŪÓŠ £ØČĪŠ“Ņ»Ģõ£©”£
£Ø4£©ČōĶł·“Ó¦µÄČŻ»żÖŠ¼ÓČė1.5molH2 ŗĶ1.0molCO£¬ŌŚĒśĻßZ¶ŌÓ¦ĪĀ¶ČĻĀ·“Ó¦“ļĘ½ŗā”£ĄūÓĆÉĻŹöĶ¼ÖŠaµć¶ŌÓ¦µÄCOĘ½ŗā×Ŗ»ÆĀŹ£¬¼ĘĖć2H2(g)£«CO(g) CH3OH(g)µÄĘ½ŗā³£Źż”££ØŠ“³ö¼ĘĖć¹ż³Ģ£©
CH3OH(g)µÄĘ½ŗā³£Źż”££ØŠ“³ö¼ĘĖć¹ż³Ģ£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com