
��ʢ��������ˮ�Ҵ����Թ��У�����һС�����еġ����ɱ���ú�͵Ľ����ƣ�Ѹ�������е��ܵĵ�������ס�Թܿڣ���һС�Թ��ռ����鴿�����ȼ�����Ѹ����С�ձ����ڻ����ϣ�Ƭ�̣�Ѹ�ٵ�ת�ձ������ձ��м�����������ʯ��ˮ��

�۲���������±���
|
�Ҵ����Ʒ�Ӧ������ |
����ȼ�յ����� |
������� |
|
|
����ȼ��ʱ����� �� С�ձ��ڱ� �� ����ʯ��ˮ �� |
��Ӧ��ֻ������
|
д���Ҵ����Ʒ�Ӧ�Ļ�ѧ����ʽ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ����̽���Ҵ��ķ��ӽṹ��ij��ѧ����С�����������ʵ�鷽������ʢ��������ˮ�Ҵ����Թ��У�����һ������ú�͵Ľ����ƣ����Թܿ�Ѹ����������ҽ��ע����ͷ�ĵ���������ȼ�ų������壬����һ�����С�ձ����ڻ����ϣ���ͼ�������ձ����ϳ���Һ�κ�Ѹ�ٵ�ת�ձ������ձ��м��������ij���ʯ��ˮ���۲������ǣ�
Ϊ����̽���Ҵ��ķ��ӽṹ��ij��ѧ����С�����������ʵ�鷽������ʢ��������ˮ�Ҵ����Թ��У�����һ������ú�͵Ľ����ƣ����Թܿ�Ѹ����������ҽ��ע����ͷ�ĵ���������ȼ�ų������壬����һ�����С�ձ����ڻ����ϣ���ͼ�������ձ����ϳ���Һ�κ�Ѹ�ٵ�ת�ձ������ձ��м��������ij���ʯ��ˮ���۲������ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
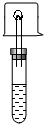 ��ʢ��������ˮ�Ҵ����Թ��У�����һС�����еġ����ɱ���ú�͵Ľ����ƣ�Ѹ�������е��ܵĵ�������ס�Թܿڣ���һС�Թ��ռ����鴿�����ȼ�����Ѹ����С�ձ����ڻ����ϣ�Ƭ�̣�Ѹ�ٵ�ת�ձ������ձ��м�����������ʯ��ˮ���۲���������±���
��ʢ��������ˮ�Ҵ����Թ��У�����һС�����еġ����ɱ���ú�͵Ľ����ƣ�Ѹ�������е��ܵĵ�������ס�Թܿڣ���һС�Թ��ռ����鴿�����ȼ�����Ѹ����С�ձ����ڻ����ϣ�Ƭ�̣�Ѹ�ٵ�ת�ձ������ձ��м�����������ʯ��ˮ���۲���������±���| �Ҵ����Ʒ�Ӧ������ | ����ȼ�յ����� | ������� |
Na�����Թܵײ� Na�����Թܵײ� ��Ӧ�����ݲ��� ��Ӧ�����ݲ��� ��Ӧʱ�ų����� ��Ӧʱ�ų����� |
����ȼ��ʱ����� ��ɫ ��ɫ ��С�ձ��ڱ� ��ˮ�飨����ˮ���� ��ˮ�飨����ˮ���� ����ʯ��ˮ ������� ������� �� |
��Ӧ��ֻ������ H2 H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����A��B�⣬��ѡһ����⣬���ⶼ��������A��Ϊ��B������ף�
�����A��B�⣬��ѡһ����⣬���ⶼ��������A��Ϊ��B������ף�| �Ҵ����Ʒ�Ӧ������ | ����ȼ�յ����� | ������� |
| �� Na�����Թܵײ� Na�����Թܵײ� �� ��Ӧ�����ݲ��� ��Ӧ�����ݲ��� �� ��Ӧʱ�ų����� ��Ӧʱ�ų����� |
��ɫ ��ɫ ��ˮ�飨����ˮ���� ��ˮ�飨����ˮ���� ������� ������� |
H2 H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ����̽���Ҵ������ʣ�ij��ѧ����С��ͨ���������ϲ������ʵ�鷽������̽����
Ϊ����̽���Ҵ������ʣ�ij��ѧ����С��ͨ���������ϲ������ʵ�鷽������̽����| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(6�֣���ʢ��������ˮ�Ҵ����Թ��У�����һС�����еġ����ɱ���ú�͵Ľ����ƣ�Ѹ�������е��ܵĵ�������ס�Թܿڣ���һС�Թ��ռ����鴿�����ȼ�����Ѹ����С�ձ����ڻ����ϣ���Ƭ�̣�Ѹ�ٵ�ת�ձ������ձ��м�����������ʯ��ˮ���۲���������±���

|
�Ҵ����Ʒ�Ӧ������ |
����ȼ�յ����� |
���� |
|
�� ��
|
����ȼ��ʱ����� �� С�ձ��ڱ� �� ����ʯ��ˮ �� |
�������Ϊ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com