
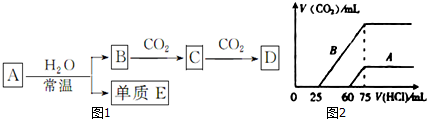
·ÖĪö £Ø1£©Ģ¼ĖįøĘ²»ČÜÓŚĖ®£»
£Ø2£©ĄūÓĆÅØĮņĖįøÉŌļŃõĘų£»
£Ø3£©µā¼ÓČČŅ×Éż»Ŗ£»
£Ø4£©Ģ¼ĖįøĘ¼ÓČČ·Ö½āÉś³ÉCaO£»
£Ø5£©ĻõĖį¼ŲŗĶĀČ»ÆÄĘČܽā¶ČŹÜĪĀ¶ČÓ°Ļģ²»Ķ¬£®
½ā“š ½ā£ŗ£Ø1£©æÉÓĆ¹żĀĖµÄ·½·Ø³żČ„Ca£ØOH£©2ČÜŅŗÖŠŠüø”µÄCaCO3Ī¢Į££¬¹Ź“š°øĪŖ£ŗA£»
£Ø2£©³żČ„O2ÖŠÉŁĮæµÄĖ®ÕōĘų£¬ÓĆŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘæĻ“Ęų³żŌÓ£¬¹Ź“š°øĪŖ£ŗD£»
£Ø3£©µā¼ÓČČŅ×Éż»Ŗ£¬ŌņĄūÓĆÉż»Ŗ·ØµĆµ½µā£¬¹Ź“š°øĪŖ£ŗB£»
£Ø4£©Ģ¼ĖįøĘ¼ÓČČ·Ö½āÉś³ÉŃõ»ÆøĘ£¬æÉÓĆ¼ÓČČ·Ö½ā·Ø·ÖĄė£¬¹Ź“š°øĪŖ£ŗC£»
£Ø5£©ĻõĖį¼ŲŗĶĀČ»ÆÄĘČܽā¶ČŹÜĪĀ¶ČÓ°Ļģ²»Ķ¬£¬ŌņĄūÓĆÖŲ½į¾§·Ø“ÓĻõĖį¼ŲŗĶĀČ»ÆÄʵĻģŗĻŅŗÖŠ»ńµĆĻõĖį¼Ų£¬¹Ź“š°øĪŖ£ŗF£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄ·ÖĄė”¢Ģį“æ·½·Ø¼°Ń”Ōń£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĪļÖŹµÄŠŌÖŹ¼°·ÖĄėŌĄķĪŖ½ā“šµÄ¹Ų¼ü£¬×¢ŅāĪļÖŹŠŌÖŹ²īŅģ¼°·ÖĄė·½·ØµÄŃ”Ōń£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na”śNaOH”śNa2CO3”śNaCl | B£® | Al”śAl2O3”śAl£ØOH£©3”śAlCl3 | ||
| C£® | Mg”śMgCl2”śMg£ØOH£©2”śMgSO4 | D£® | Fe”śFeCl2”śFe£ØOH£©2”śFe£ØOH£©3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 16g CH4ÖŠŗ¬ÓŠ4NAøöŌ×Ó | |
| B£® | 1mol/L NaClČÜŅŗŗ¬ÓŠNAøöNa+ | |
| C£® | 1mol AlŗĶ×ćĮæĻ”ŃĪĖį·“Ó¦×ŖŅĘ3NAøöµē×Ó | |
| D£® | ±ź×¼×“æöĻĀ£¬22.4L CCl4ÖŠŗ¬ÓŠ4NAøöĀČŌ×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆ·“Ó¦µÄŃõ»Æ¼ĮĪŖKMnO4 | |
| B£® | ·¢Éś»¹Ō·“Ó¦µÄŹĒH2C2O4 | |
| C£® | øĆĄė×Ó·½³ĢŹ½ÓŅ²ą·½æņÄŚµÄ²śĪļŹĒH2O | |
| D£® | 6mol H+²Ī¼Ó·“Ó¦Ź±£¬µē×Ó×ŖŅĘ10mol |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
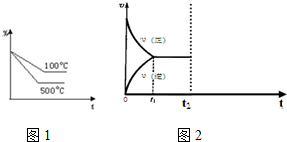
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2NO2£Øg£©?N2O4£Øg£©£¬ÉżĪĀŹ±ĢåĻµŃÕÉ«¼ÓÉīĖµĆ÷Õż·“Ó¦ĪŖĪüČČ·“Ó¦ | |
| B£® | N2£Øg£©+3H2£Øg£©?2NH3£Øg£©£¬ČōÉżĪĀŹ±NH3µÄÅØ¶Č¼õŠ”£¬ŌņĘ½ŗā³£ŹżKŌö“ó | |
| C£® | CO£Øg£©+H2O£Øg£©?CO2£Øg£©+H2£Øg£©£¬ÉżĪĀŹ±COµÄ×Ŗ»ÆĀŹŌö“ó£¬ĖµĆ÷Õż·“Ó¦ĪŖĪüČČ·“Ó¦ | |
| D£® | ·“Ó¦2HI£Øg£©?H2£Øg£©+I2£Øg£©“ļµ½Ę½ŗāŗó£¬Ōö“óHIµÄĪļÖŹµÄĮæ£¬Ę½ŗā²»ŅĘ¶Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

 C6H14
C6H14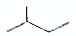 C5H12
C5H12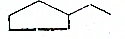 C7H14
C7H14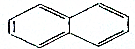 C10H8£®
C10H8£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com