
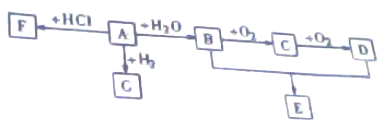
| Cu |
| �� |
| Ũ���� |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe��FeCl3��Һ��Ӧ��Fe+Fe3+=2Fe2+ |
| B����AlCl3��Һ�е��백ˮ��Al3++3OH-=Al��OH��3�� |
| C������ϡ���ᷴӦ��Fe+2H+=Fe2++H2�� |
| D���Ȼ��������ᷴӦ��Ba2++SO42-=BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ��������ͼװ����ȡ����������
ʵ��������ͼװ����ȡ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
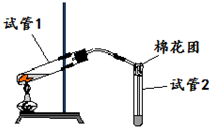 ��ͼ��ʵ������ͭƬ��Ũ������ȡSO2����֤�����ʵ�װ��ͼ�����Թ�1�м���һС��ͭƬ���ټ���3��5mLŨ���ᣬ�ô����ܵĵ������������Թܣ����ȣ������ɵ�����ͨ���Թ�2����Һ�У��ش��������⣺
��ͼ��ʵ������ͭƬ��Ũ������ȡSO2����֤�����ʵ�װ��ͼ�����Թ�1�м���һС��ͭƬ���ټ���3��5mLŨ���ᣬ�ô����ܵĵ������������Թܣ����ȣ������ɵ�����ͨ���Թ�2����Һ�У��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Al��OH��3 |
| B��FeCl2 |
| C��CuS |
| D��H2SiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
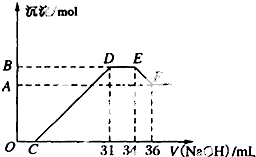 ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺
ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Σ��ڷ�Ӧ�����Һ�У���μ���4mol?L-1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com