
���� 2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O�У�MnԪ�صĻ��ϼ���+7�۽���Ϊ+2�ۣ�ClԪ�صĻ��ϼ���-1������Ϊ0���÷�Ӧ��ת��10e-��������ԭ��Ӧ���������������Դ�����������������ԣ�
KMnO4��������100mL 0.5mol��L-1����Һ����Ҫ100mL����ƿ����ͷ�ιܣ������n=cV��m=nM��c=$\frac{n}{V}$���
��� �⣺��1��MnԪ�صĻ��ϼ���+7�۽���Ϊ+2�ۣ�ClԪ�صĻ��ϼ���-1������Ϊ0���÷�Ӧ��ת��10e-����Ӧ��ת��10�����ӣ���˫���ű�ʾ����ת�Ʒ�������ĿΪ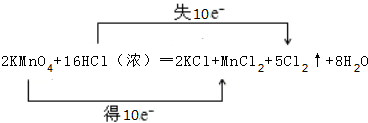 ��
��
�ʴ�Ϊ�� ��
��
��2��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O��ͨ�����ϼ������жϣ�MnԪ����+7�۽��͵�+2�ۣ�ClԪ�صĻ��ϼ���-1�����ߵ�0��2mol������صõ�������������10molHClʧ�����ǻ�ԭ���������������뻹ԭ�������ʵ���֮����2��10=1��5��
�ʴ�Ϊ��1��5��
��3�����������������Դ�����������������Կ�֪��������ΪKMnO4��Cl2���ʴ�Ϊ��ǿ��
��4���ɷ�Ӧ��֪��ת��10e-����5molCl2����Ӧ��ת����2mol���ӣ��������Cl2�ڱ�״�������Ϊ1mol��22.4L/mol=22.4L���ʴ�Ϊ��2.24��
��5����KMnO4��������100mL 0.5mol��L-1����Һ����Ҫ100mL����ƿ��������Ҫ��ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܣ�100mL����ƿ��
����Ҫ���������Ϊ0.1L��0.5mol/L��158g/mol=7.9g���ʴ�Ϊ��7.9��
��A����ˮ����ʱ���ӿ̶��ߣ���Һ�����ƫС����Ũ��ƫ��A��ѡ��
B������ƿ�ڱڸ���ˮ���δ���ﴦ�����������趨�ݼ�ˮ����ʵ����Ӱ�죬��B��ѡ��
C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���ϣ���Һ���ƫ����Ũ��ƫС����Cѡ��
D�����ܽ������������Һ�彦���ձ��⣬���ʼ��٣���������ҺŨ��ƫС����Dѡ��
�ʴ�Ϊ��CD��
���� ���⿼��������ԭ��Ӧ����Һ�����ƣ�Ϊ��Ƶ���㣬���շ�Ӧ��Ԫ�صĻ��ϼ۱仯����Һ���Ʋ���Ϊ���Ĺؼ������ػ������ת�Ƶ��Ӽ�ʵ����������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
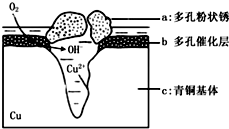 ��ͼΪ��ͭ���ڳ�ʪ�����з����绯ѧ��ʴ��ԭ��ʾ��ͼ�������е� Cl- ��ɢ���ڣ�������缫�����������ɶ��״�� Cu2 �� OH ��3 Cl������˵����ȷ���ǣ�������
��ͼΪ��ͭ���ڳ�ʪ�����з����绯ѧ��ʴ��ԭ��ʾ��ͼ�������е� Cl- ��ɢ���ڣ�������缫�����������ɶ��״�� Cu2 �� OH ��3 Cl������˵����ȷ���ǣ�������| A�� | ��ʴ�����У������� b �� c | |
| B�� | �� �� Cu 2�� OH ��3 Cl �� ���ӷ���ʽΪ��2Cu2++3OH -�TCu 2�� OH �� 3+ | |
| C�� | ������ 4.29gCu 2�� OH ��3 Cl�������������ı�״���������Ϊ0.448L | |
| D�� | �����ĵ缫��ӦʽΪ��O 2-4e-+2H+�T2OH - |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ũ������������������������ʵ��˵��������������Ũ���ᷢ����Ӧ | |
| B�� | �������ƿ���Ϊ������ߺ�DZˮͧ�ڵĹ����� | |
| C�� | ����ϡ�������ͭп�Ͻ��Ƴɵļٽ�� | |
| D�� | ���������ڵ�̲��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
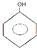 ����������
���������У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
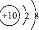 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ������ | ������ |
| A | SO2��Ư���� | SO2��ʹ��ˮ��ɫ |
| B | SiO2�е����� | SiO2�������Ʊ����ά |
| C | Fe3+��ǿ������ | FeCl3��Һ�����ڻ��շϾɵ�·���е�ͭ |
| D | Ũ������ǿ������ | Ũ��������ڸ���H2��CO |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com