
 ��
������ ��1��Cuԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��ʧȥ4s�ܼ�1�������γ�Cu+��
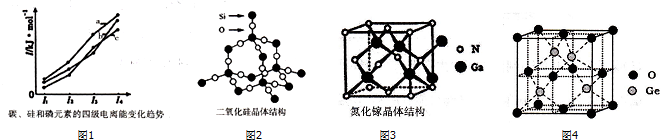
��2��ͬ�������϶��µ�һ�����ܼ�С��PԪ��3p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ���Si�ĵ�һ��������С��Pԭ�ӵ��ĵ�����Ϊʧȥ4s2����1�����ӣ�Ϊȫ���ȶ�״̬����������������ϴ�
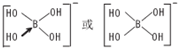
��3���ڶ������辧�壬���Կ����ھ������ÿ��Si-Si��֮������Oԭ�ӣ�����Si��ÿ��Siԭ���γ�4��Si-Si������ͼ��֪ÿ2��Si-Si�������γ�2����Ԫ������4��Si-Si������2�������γ�6����ϣ�
��4����ѹ�£������أ�GaN���ľ����۵�1700�棬���侧������Ϊԭ�Ӿ��壬������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
��5��[B��OH��4]-��Bԭ�ӹµ��Ӷ���=$\frac{3+1-1��4}{4}$=0���ӻ������ĿΪ4��[B��OH��4]-��Bԭ����Oԭ��֮���γ�4�����ۼ�������1��Ϊ��λ����
��6�����ݾ�̯�����㾧����Ge��Oԭ����Ŀ����������ԭ��������ʾ�����������ٽ��m=��V���㣮
��� �⣺��1��Cuԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��ʧȥ4s�ܼ�1�������γ�Cu+��Cu+��̬ʱ�����Ų�ʽΪ1s22s22p63s23p63d10�������ռ�ݵ�14ԭ�ӹ����
�ʴ�Ϊ��1s22s22p63s23p63d10��14��
��2��ͬ�������϶��µ�һ�����ܼ�С��PԪ��3p�ܼ�Ϊ�����ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ���Si�ĵ�һ��������С����ͼ�е�һ�����ܿ�֪��cΪSi��Pԭ�ӵ��ĵ�����Ϊʧȥ4s2����1�����ӣ�Ϊȫ���ȶ�״̬����������������ϴ�֪bΪP��aΪC��
�ʴ�Ϊ��b��
��3���ڶ������辧�壬���Կ����ھ������ÿ��Si-Si��֮������Oԭ�ӣ�����Si��ÿ��Siԭ���γ�4��Si-Si������ͼ��֪ÿ2��Si-Si�������γ�2����Ԫ������4��Si-Si������2�������γ�6����ϣ���ÿ��Siԭ������ʮ��Ԫ����ĿΪ6��2=12��
�ʴ�Ϊ��12��
��4����ѹ�£������أ�GaN���ľ����۵�1700�棬���侧������Ϊԭ�Ӿ��壬������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
�ʴ�Ϊ��ԭ�Ӿ��壻������1��Ga��4��Nԭ�����ϣ���Gaԭ���к���3���۵��ӣ�Ga�ṩ1���չ����Nԭ���ṩ�ŶԵ����γ���λ����
��5��[B��OH��4]-��Bԭ�ӹµ��Ӷ���=$\frac{3+1-1��4}{4}$=0���ӻ������ĿΪ4��Bԭ�Ӳ�ȡsp3�ӻ���[B��OH��4]-��Bԭ����Oԭ��֮���γ�4�����ۼ�������1��Ϊ��λ�����ýṹ��ʽ��ʾΪ ��
��
�ʴ�Ϊ��sp3�ӻ��� ��
��
��6��������Geԭ����ĿΪ4��Oԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ѧʽΪGeO����������ԭ������ΪM����;�������Ϊ��$\frac{4����M+16��}{6.02��1{0}^{23}}$g����$\frac{4����M+16��}{6.02��1{0}^{23}}$g=7.4g��cm-3����4.3��l0-8 cm��3�����M=72.5��
�ʴ�Ϊ��GeO��72.5��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų��������ܡ������ṹ����㡢�����ӻ�����ȣ���2��ע����ݺ��ع���������������ܾ������⣬��3��Ϊ�״��㣬��Ҫѧ���߱�һ���Ŀռ�������۲�������


 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ������ƿǰ�������Ƿ�©ˮ | |
| B�� | ������ˮע������ƿ�У�Һ����̶�����1-2cmʱ�����ý�ͷ�ιܵμ���Һ����̶������� | |
| C�� | ������Һʱ������Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���̶��� | |
| D�� | ���ݺ�Ǻ�ƿ�����������µߵ���ҡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 89.6 mL | B�� | 112 mL | C�� | 168 mL | D�� | 224 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ${\;}_{81}^{203}$Tl��${\;}_{81}^{205}$Tl��������ͬ | |
| B�� | ${\;}_{81}^{203}$Tl��${\;}_{81}^{205}$Tl��Ϊͬ�������� | |
| C�� | ${\;}_{81}^{203}$Tl��${\;}_{81}^{205}$Tl��Ϊͬλ�� | |
| D�� | ${\;}_{81}^{203}$Tl��${\;}_{81}^{205}$Tl�����ֺ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Sn2+��Fe2+��Ce3+ | B�� | Fe2+��Ce3+��Sn2+ | C�� | Fe2+��Sn2+��Ce3+ | D�� | Ce3+��Fe2+��Sn2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢݢ� | B�� | �٢ܢ� | C�� | �ڢۢܢ� | D�� | �ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com