
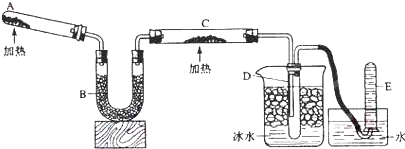
��12�֣�����֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��

�ش��������⣺
��1��A�м���Ĺ�̬������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2��B���������� ���������� ��
��3��ʵ��ʱ�ڹ۲쵽C�е������� ���������������������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4��ʵ��ʱ��D�й۲쵽�������� ��D���ռ����������� ������������е�ijһ�����ʵķ����������� ��
 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����Ӧ����ʽΪ
![]() 2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
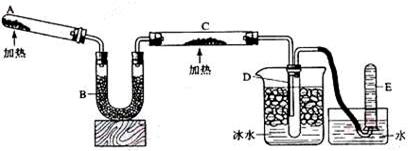
��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
���鰱��ͨ�����õķ�����________ _����������__ ________��
��2��B���������� ���������� ��
��3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______������������ԭ������
��4��ʵ��ʱ��D���ռ�����Һ̬������ ��E���ռ�����������__________��
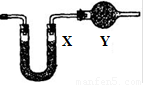
��5����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D��װ��ˮ���ձ����ָij���ͼװ�ã�U�ι�X��װ��____________��������________________�������Y��װ�м�ʯ�ң�������_______________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣���֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����Ӧ����ʽΪ
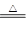 2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
���鰱��ͨ�����õķ�����________ _����������__ ________��
��2��B���������� ���������� ��
��3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______������������ԭ������
��4��ʵ��ʱ��D���ռ�����Һ̬������ ��E���ռ�����������__________��
��5����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D��װ��ˮ���ձ����ָij���ͼװ�ã�U�ι�X��װ��____________��������________________�������Y��װ�м�ʯ�ң�������_______________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com