
���� ��1��������ȼ�յ�ͨʽ400��ʱ�����ɵ�ˮΪ��̬��������ȼ��ͨʽCxHy+��x+$\frac{y}{4}$��O2$\stackrel{��ȼ}{��}$xCO2+$\frac{y}{2}$H2O��������������������㣻
��2������3LA��ȫȼ����������������ж�x�ķ�Χ��Ȼ��д����A�ķ���ʽ��
��3����A�ڳ�����Ϊ��̬����x��4��Ȼ�����3LA��ȫȼ��������������ȷ��m�ķ�Χ��
��� �⣺��1�������ķ���ʽΪCxHy����
CxHy+��x+$\frac{y}{4}$��O2$\stackrel{��ȼ}{��}$xCO2+$\frac{y}{2}$H2O��g�� �������������V
1 ��x+$\frac{y}{2}$-x-$\frac{y}{4}$-1��=$\frac{y}{4}$-1
3L ��m+6-m-3��L
��ã�y=8��
�������ķ���ʽΪ��CxH8��˵����A�����Ӧ����������ÿ�������к�8����ԭ�ӣ�
�ʴ�Ϊ����A�����Ӧ����������ÿ�������к�8����ԭ�ӣ�
��2����m=27ʱ��3L��A��ȫȼ��������27L��������x+$\frac{8}{4}$��$\frac{27L}{3L}$=9������x��7��������A�ķ���ʽ����Ϊ��C3H8��C4H8��C5H8��C6H8��C7H8��
�ʴ�Ϊ��C3H8��C4H8��C5H8���� C6H8��C7H8����
��3������A�ڳ��³�ѹ��Ϊ��̬����ʱ��CxH8�е�x��������x��4����x+$\frac{8}{4}$��$\frac{mL}{3L}$����ã�m��3x+6������m��18��
�ʴ�Ϊ��m��18��
���� ���⿼���л������ʽȷ���ļ��㣬Ϊ�߿��ij������ͣ������е��Ѷȵ����⣬����Ĺؼ��Ǹ����л����ȼ��ͨʽ��Ȼ��������������ü��ɣ�������ض�ѧ�����������ͷ�����ָ����ѵ��������������ѧ�������������������ѧ����Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� AgNO3 ��Һ�����ɰ�ɫ��������ϡ�����������ʧ����ȷ���� Cl- ���� | |
| B�� | �� BaCl2 ��Һ�����ɰ�ɫ��������ϡ�����������ʧ����ȷ���� SO42- ���� | |
| C�� | �����ᣬ���ɵ�������ʹ����ʯ��ˮ����ǣ���ȷ���д���CO32-���� | |
| D�� | ͨ�� Cl2 ����Һ��Ϊ��ɫ�����������Һ����Һ��������ȷ����I- ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
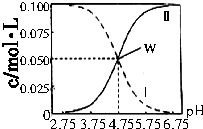 �����������ڴ���ʹ����ƵĻ����Һ�У���c��CH3COOH��+c��CH3COO-��=0��lmol•L-1ʱ��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й�������ȷ���ǣ�������
�����������ڴ���ʹ����ƵĻ����Һ�У���c��CH3COOH��+c��CH3COO-��=0��lmol•L-1ʱ��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������й�������ȷ���ǣ�������| A�� | W������ʾ����Һ�У�c��Na+��+c��H+��=c��CH3COOH��+c��OH-�� | |
| B�� | pH=5.5����Һ�У�c��CH3COOH����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | pH=3.5����Һ�У�c��Na+��+c��H+��+c��OH-��+c��CH3COOH��=0.1mol��L-l | |
| D�� | ��ʾCH3COO-Ũ�ȱ仯�������ߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ������ϩ | B�� | һ���м��� | C�� | һ��û�м��� | D�� | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2�����ģ�ͣ� | B�� | ����ĽṹʽCH3-CH3 | ||
| C�� | ������Ľṹ��ʽ��C��CH3��4 | D�� | CCl4�ĵ���ʽ��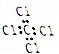 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
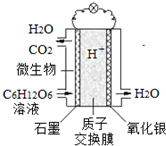 �����ѧ�����Ƶ�һ�����͡������ء����Խ���ˮ�е��л���ת��Ϊ���ܣ���ԭ��ʾ����ͼ�������йظõ�ص�˵����ȷ���ǣ�������
�����ѧ�����Ƶ�һ�����͡������ء����Խ���ˮ�е��л���ת��Ϊ���ܣ���ԭ��ʾ����ͼ�������йظõ�ص�˵����ȷ���ǣ�������| A�� | ��ع���ʱ��H+��ʯī�缫�ƶ� | |
| B�� | ʯī�缫�Ϸ�ӦΪ��C6H12O6+6H2O-24e-=6CO2��+24H+ | |
| C�� | �������缫�Ϸ�ӦΪ��Ag2O+2e-=2Ag+O2- | |
| D�� | �õ��ÿת��4mol���ӣ�ʯī�缫����33.6 L CO2���壨��״���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com