
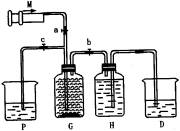
��2��β������ʱ�رյ��ɼ�a�͵��ɼ�________�����ɼ�________��
��3������β��ʱ��������Ӧ�Ļ�ѧ����ʽ��________��
��4���ú�0.032molHCl��Ũ����������ĸ�����ع��巴Ӧ���������������ʵ���Ӧ________������ڡ����ڻ�С�ڡ���0.0lmol��
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
��2003���Ͼ�����������⣩����˵���У���ȷ����
( )
A. ���³�ѹ�£�1mol�������еĺ����������20NA
B. 32g������32g����������ԭ������Ϊ2NA
C. 18gˮ�������ĵ�������8NA
D. ��״���£�1L������ȫȼ�պ����ɶ�����̼�ķ�������5NA��22.4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�013
( )
A. C1����N0![]() ��Ba2����Ca2��
��Ba2����Ca2��
B.S0![]() ��C H3COO����Al3����Mg2��
��C H3COO����Al3����Mg2��
C. Na����K����SO![]() ��Cl��
��Cl��
D. NH![]() ��Ba2����C1����HCO
��Ba2����C1����HCO![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�013
( )
A. ���³�ѹ�£�1mol�������еĺ����������20NA
B. 32g������32g����������ԭ������Ϊ2NA
C. 18gˮ�������ĵ�������8NA
D. ��״���£�1L������ȫȼ�պ����ɶ�����̼�ķ�������5NA��22.4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
��2003���Ͼ�����������⣩ij���������ȷֽ����ɽ�����������������������������ɵĶ������������������ʵ���֮��Ϊ8��1�������Ԫ�صĻ��ϼ��ڷ�Ӧ�����еı仯��
( )
A. ����
B. ����
C. ����
D. ��ȷ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com