
����ѪҺ��Ca2+��Ũ��һ�����g/cm3����ʾ(��1 cm3Ѫ���к��е�Ca2+������)����ȡ һ�������Ѫ�����������IJ���泥�(NH4)2C2O4����Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵ��������(H2C2O4)������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ�
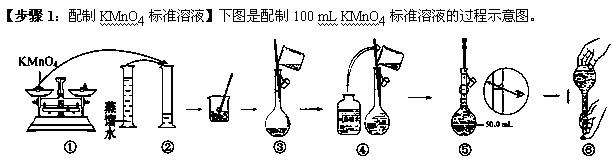 (1)����۲�ͼʾ�жϣ����в���ȷ����������� (�����)��
(1)����۲�ͼʾ�жϣ����в���ȷ����������� (�����)��
(2)����ȷ��100 mL��Һ�����������__________________
(3)�����ͼʾ�IJ��������Ƶ���Һ����ʵ�飬������������ȷ������£������Ƶ���ҺŨ�Ƚ�
______(�ƫ��ƫС��)��
������2���ⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ���ȡѪ��20.00 mL����������������õ����ᣬ����0.020 mol/L ����KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ�����ʱ������12.00 mL KMnO4��Һ��
(4)д������������KMnO4��Һ��Ӧ�����ӷ���ʽ ��
(5)�ζ����յ�Ϊ ��
(6)�������㣬ѪҺ��Ʒ��Ca2+��Ũ��Ϊ__________g/cm3��
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com