
��ҵ��NaCl��NH3��CO2��Ϊԭ�����Ƶ�NaHCO3���������������
��1��д����ʵ���Ʊ�����ķ�Ӧ����ʽ �� ��
��2��̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����
������ĸ��ţ���a��̼������������ˮ b��̼�����������ֽ�
c��̼�����Ƶ��ܽ����Խ�С����������Һ�����Ƚᾧ����
d��̼�����Ƶ��ȶ��Դ���̼����
��3��ij�С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�顣
�� һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��
װ������ͼ��ʾ��ͼ�мг֡��̶��õ�����δ���������Իش������й����⣺
������װ���е��Լ��� ���������� ��
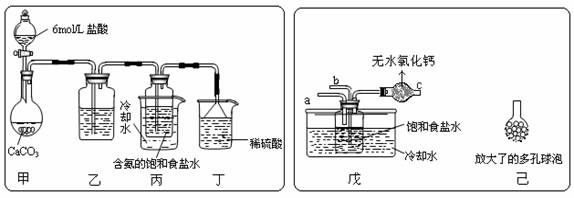 ����װ����ϡ����������� ��
����װ����ϡ����������� ��
����ʵ����������NaHCO3 ����IJ����� ���������������ƣ�,�ò�������Ҫ�IJ���������
�� ��һλͬѧ��ͼ����װ�ã�����װ��δ����������ʵ�顣
����ʵ��ʱ�����ȴ�a��ͨ��_______���壬˵��ԭ��_________________________
������ͬѧ��������װ�õ�b���¶����Ӽ�װ�ã�������
��4�� ̼�������������ù���12.28g��������ʯ��ˮ��ַ�Ӧ�����ó�����ϴ
�ӡ���������Ϊ12.00g,�����ù�����̼���Ƶ���������Ϊ_______��
��1��NaCl + CO2 +NH3+H2O=NaHCO3+NH4Cl
2NaHCO3=Na2CO3+CO2+H2O��2�֣�
��2��c��1�֣�
��3���٣�����̼��������Һ����1�֣���ȥCO2�е�HCl���壨1�֣�
��������δ��Ӧ���NH3��1�֣����𡰷�ֹ������������CO2�������֣���
�����ˣ�1�֣� ��������©�����ձ���2�֣�
�ڣ������� ���������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����李�����2�֣�
����������������Һ�Ӵ���������CO2�������ʡ���2�֣�
��4��86.3 %����0.863����0.86Ҳ�÷֣���2�֣�


 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��X��Y��Z��N���ֶ�����Ԫ�ص�ԭ�������������� ������Yԭ�ӵ�L���Ӳ��У��ɶԵ�����δ�ɶԵ���ռ�ݵĹ������ȣ����չ����Xԭ�ӵ�L���Ӳ���δ�ɶԵ�������Y��ͬ�������пչ����W��Z��ԭ���������10����Zԭ�ӵĵ�һ��������ͬ��������С��N�ǵؿ��к������Ľ�����
������Yԭ�ӵ�L���Ӳ��У��ɶԵ�����δ�ɶԵ���ռ�ݵĹ������ȣ����չ����Xԭ�ӵ�L���Ӳ���δ�ɶԵ�������Y��ͬ�������пչ����W��Z��ԭ���������10����Zԭ�ӵĵ�һ��������ͬ��������С��N�ǵؿ��к������Ľ�����
(1)д������Ԫ�ص�Ԫ���� �ƣ�X ��Y ��Z ��
�ƣ�X ��Y ��Z ��
(2)��XY���ӻ�Ϊ�ȵ������� ��XY���ӵĵ���ʽΪ �����ݵ������ص���ʽ�IJ�ͬ��
�����ﹲ�ۼ�����Ҫ������ �����ֵΪ ��
XY2��ZYW��Һ��Ӧʱ��ͨ�����Ʒ�Ӧ������ʵ���֮�ȣ����Եõ���ͬ�IJ����ͬ�����£���ˮ
���ܽ�Ƚ�С�IJ����� (д��ѧʽ)��
(4) �õ���ʽ��ʾZ2Y���γɹ��� (2��)��
(5) Z��N����Ԫ�ص�����������Ӧˮ���ﷴӦ�����ӷ���ʽΪ ��2�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ���������ϴ�������ͼ��ʾ��ϵ��

�����и������ϵ��˵������ȷ���ǣ� ��
A�����������������ڰ�����ϵ B�����������������ڰ�����ϵ
C�������뻯�������ڽ����ϵ D��������ԭ��Ӧ�뻯�Ϸ�Ӧ���ڲ��й�ϵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д���з�Ӧ����ʽ��
��1�����������Կ�ʴ��������д���䷴Ӧ�Ļ�ѧ����ʽ��
��
��2�������������ڿ����о��ñ���������д���䷴Ӧ�Ļ�ѧ����ʽ��
��
��3�������������������ڹ�������������Һ����д���䷴Ӧ�����ӷ���ʽ��
��
��4�����������Һ��ͨ�������CO2���壬��д���䷴Ӧ�����ӷ���ʽ��
��
��5����ͭƬ������FeCl3��Һ�У���д���䷴Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ����
A. ��������������Fe2O3+6H+ �� 2Fe3++3H2O
B. �����ʵ�����KHCO3��Ba(OH)2����Һ��ϣ�
HCO3��+ Ba2++OH�� �� BaCO3�� + H2O
C. NaHSO3��Һ�����Ե�ԭ���ǣ�HSO3�� �� SO32�� + H+
D. ����ˮ��Һ�ʼ��Ե�ԭ���ǣ�S2�� + 2H2O  H2S + 2OH��
H2S + 2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л����A��Ϊͬ���칹��, ���ⶨ���ǵ���Է�������С��100����1mol����O2�г��ȼ�յõ������ʵ�����CO2��H2O (g ) ��ͬʱ����112LO2����״�����������������½�1 mol����ȫˮ���������1 mol �� ��1mol�� ��������һ�������£������Ա�����������Ϊ�ҡ�
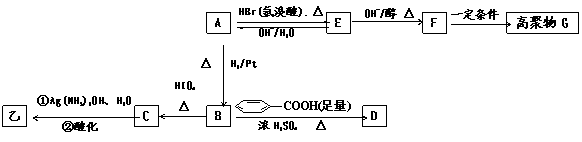

��������ײⶨ���ڼ�A�Ľṹ�ж�����C=O˫����C-O������B��
HIO4���ڲ�����ʱֻ����һ�ֲ���C������Ϊ����ط�Ӧ����Ϣ��ת����
ϵ��

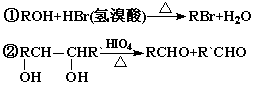
��1����ȷ����д���ķ���ʽ �������ͬ�����ʵ�ͬ���칹�干�� �֣������ף���
��2��E �� F �ķ�Ӧ����Ϊ ��Ӧ ��
��3��A �Ľṹ��ʽΪ ��G �Ľṹ��ʽΪ ��
��4��B ��D�ķ�Ӧ��ѧ����ʽΪ�� ��
��5��д��C���������½��з�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͨ�����ǰѲ�1molij��ѧ�����յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ����Ҳ���Թ��ƻ�ѧ��Ӧ�ķ�Ӧ��(��H)����ѧ��Ӧ�Ħ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJ������һЩ��ѧ���ļ��ܡ�
| ��ѧ�� | C��H | C��F | H��F | F��F |
| ����/(kJ��mol��1) | 414 | 489 | 565 | 155 |
���ݼ������ݹ������з�ӦCH4(g)��4F2(g)===CF4(g)��4HF(g)�ķ�Ӧ�Ȧ�HΪ]
A.��1940kJ��mol��1 B.1940kJ��mol��1 C.��485kJ��mol��1 D.485kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���仯�У�����һ������ǰ�ߵ��ǣ� ���� 
A. ��ѧ�仯�������仯 B. ������ԭ��Ӧ�����ֽⷴӦ
C. ������ԭ��Ӧ�����Ϸ�Ӧ D. �кͷ�Ӧ�����ֽⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA���������ӵ�����������������ȷ����
A����״���£�11.2L��O2��NO�Ļ���ﺬ�еķ�����ԼΪ0.5��6.02��1023
B��1mol���ǻ���1 mol������������������������Ϊ9 NA
C�����³�ѹ��42g ��ϩ�Ͷ�ϩ��������У����Լ���Ϊ6NA
D��6.4g SO2������������Ӧ����SO3��ת�Ƶ�����Ϊ0.2NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com