
���� ��1���Ȼ�������ǿ�������Σ�������ˮ�����Һ�����ԣ�25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c��OH-��=c��H+����
��2����֪NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4=Na++H++SO42-����Һ�д��ڵ���غ㣬���ݵ���غ��ж�c��H+������Դ�Сc��OH-��+c��SO42-����
����������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ���������߷�Ӧ�������ᱵ���������ƺ�ˮ����Ӧ����Һ�ʼ��ԣ�
��3���κε������Һ�ж�����ˮ�������c��OH-��=c��H+�������¶���ˮ�����ӻ�����=c��OH-����c��H+��=2��10-7��2��10-7=4��10-14��c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$��
��4���ȸ��ݸ��¶��´�ˮ�����������Ũ�ȼ����ˮ�����ӻ���Ȼ�����ˮ�����ӻ�������������������Һ��c��OH-�����ٸ����������������֮��Ĺ�ϵ����������������������֮�ȣ�
��� �⣺��1���Ȼ�������ǿ�������Σ�������ˮ�����Һ�����ԣ�25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c��OH-��=c��H+��=10-2mol/L��ˮ�����ӷ���ʽΪFe3++3H2O?Fe��OH��3�����壩+3H+��
�ʴ�Ϊ��10-2mol/L��Fe3++3H2O?Fe��OH��3�����壩+3H+��
��2��NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4=Na++H++SO42-����Һ�д��ڵ���غ�c��H+��+c��Na+��=c��OH-��+2c��SO42-���������غ�c��Na+��=c��SO42-�������Ե�c��H+��=c��OH-��+c��SO42-����
����������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ���������߷�Ӧ�������ᱵ���������ƺ�ˮ����Ӧ����ʽΪNaHSO4+Ba��OH��2=BaSO4��+NaOH+H2O����Һ�е�������NaOH������Һ�ʼ��ԣ�pH��7��
�ʴ�Ϊ��=������
��3���κε������Һ�ж�����ˮ�������c��OH-��=c��H+��=2��10-7 mol/L�����¶���ˮ�����ӻ�����=c��OH-����c��H+��=2��10-7��2��10-7=10-14��c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$=$\frac{4��1{0}^{-14}}{5��1{0}^{-6}}$mol/L=8��10-9 mol/L��
�ʴ�Ϊ��2��10-7 mol/L��8��10-9 mol/L��
��4��ij�¶��´�ˮ��c��H+��=1.0��10-6 mol•L-1�����Ը��¶���ˮ�����ӻ�Ϊ��KW=c��H+����c��OH-��=1.0��10-6��1.0��10-6=1��10-12��
��pH=8��Ba��OH��2��Һ��c��OH-��=10-4 mol/L��pH=5��ϡ������c��H+��=10-5 mol/L��
���������������Ϊx����������Ϊy��100��ĺ��£���ʹ�����ҺpH=7����Һ�ʼ��ԣ�
c��OH-��=$\frac{1{0}^{-12}}{1{0}^{-7}}$mol/L=10-5 mol/L��c��OH-��=$\frac{1{0}^{-4}x-1{0}^{-5}y}{x+y}$=10-5 mol/L�����x��y=2��9��
�ʴ�Ϊ��2��9��
���� ���⿼���������Һ�����жϡ�����ˮ���֪ʶ�㣬Ϊ��Ƶ���㣬���������ӵ��δٽ�ˮ���룬���ᡢ����������ӵ�����ˮ����������ӻ����������ӵļ��㷽�����״����ǣ�1���⣮


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ�ο���Ϊ��ζ����Ҳ����ʳƷ������ | |
| B�� | ��������ɹ㷺����ʳƷ���� | |
| C�� | ������һ�ַ�ɢϵ����ɢ���ǿ�����������̿���ַ�������ԭ��������ԭ�� | |
| D�� | �ҹ���������3D��ӡ���������ѺϽ��δΪԭ�ϣ�ͨ�������ۻ����ѻ���������ɻ��ѺϽ�ṹ��������ʱ���ý����ƻ�ԭ��Ӧ���Ȼ�������ȡ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������������Ҫ��Fe2O3��SiO2��Al2O3��MgO�����ʣ��Ʊ��������Ĺ����������£�
��������������Ҫ��Fe2O3��SiO2��Al2O3��MgO�����ʣ��Ʊ��������Ĺ����������£�
| ������ | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ | |
| B�� | ��ȡ��������ʱ����ˮ��NaOH��Һ����β�� | |
| C�� | Ϊ�ⶨNa2CO3��NaHCO3�������Na2CO3����������ȡa g�����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����bg���÷������� | |
| D�� | Ϊ�ⶨ��п��Ƥ��п���ȣ�����п��Ƥ���������ᷴӦ���������Լ���ʱȡ����ϴ�ӣ���ɣ����أ������ʱ��������ᵼ�²ⶨ���ƫС |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCO3-+H2O?H3O++CO32- | B�� | NH3+H2O?NH4++OH- | ||
| C�� | CO32-+2H2O?H2CO3+2OH- | D�� | Al3++3H2O?Al��OH��3+3H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��H2SO3��+c��H+��=c��SO32-��+c��OH-�� | B�� | c��Na+��=2c��SO32-��+c��HSO3-�� | ||
| C�� | c��Na+����c��HSO3-����c��H+����c��SO32-����c��OH-�� | D�� | c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
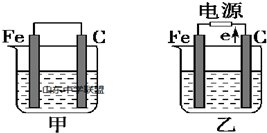
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | Zn2++2e-�TZn | B�� | Cu2++2e-�TCu | C�� | Zn-2e-�TZn2+ | D�� | 2H++2e-�TH2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com