
�������Ʊ�CuO������ͼ��
��ҵCuSO4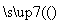
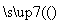 CuSO4��Һ
CuSO4��Һ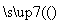 CuSO4·5H2O������������CuO
CuSO4·5H2O������������CuO
(1)������Ŀ���dz�ȥ���������ʡ�������_________________________________��
(2)������Ŀ���dz����������ǣ��μ�H2O2��Һ���Լ��ȣ���Fe2��ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Կ��� ��ҺpH��3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH��1��������ҺpH��3.5��ԭ����___________________________
��ҺpH��3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH��1��������ҺpH��3.5��ԭ����___________________________
________________________________________________________________________��
(3)������Ŀ���ǵõ�CuSO4·5H2O���塣������_____________________________
____________________________________________________________(���������)��
���ˣ�ˮԡ���Ⱥ�ɡ�ˮԡ���ȵ��ص���____________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CH4����ԭNOx�������������������Ⱦ����֪CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)����H����574 kJ��mol��1��CH4(g)��4NO(g)===2N2 (g)��CO2(g)��2H2O(g)
(g)��CO2(g)��2H2O(g)
��H����1 160 kJ��mol��1�����ڱ�״����4.48 L CH4ǡ���ܽ�һ����NO2��ԭ��N2��H2O(g)�������������зų�������Ϊ(����)
A��114.8 kJ B��232 kJ
C��368.8 kJ D��173.4 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������(KMnO4)��������(H2O2)�����ֵ��͵���������
(1)H2O2�ĽṹʽΪ________________��
(2)������������ʹ����KMnO4��Һ��ɫ����________(�����)��
A������(Na2S) B��С�մ�(NaHCO3)
C��ˮ����(Na2SiO3) D���ƾ�(C2H5OH)
(3)������ء�˫��ˮ������̿����������ˮ�Ĵ�������������������ʱ�������Ⱥ�˳��
����ϡ�����У�KMnO4��H2O2�ܷ���������ԭ��Ӧ��
������Ӧ��H2O2��2e������2H����O2��
��ԭ��Ӧ��MnO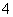 ��5e����8H������Mn2����4H2O
��5e����8H������Mn2����4H2O
д����������ԭ��Ӧ�����ӷ���ʽ��____________________________��
���ڴ�������ˮʱ������̿Ӧ�ڸ�����ط�Ӧ������Ͷ�ӣ�����ᷢ����Ӧ��KMnO4��C��H2O����MnO2��X��K2CO3(δ��ƽ)������X�Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������״��ϩ��(�����к�һ��̼̼˫��)�Ļ���������������������Ϊa����̼������������(����)
A.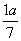 B.
B.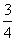 a
a
C.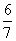 (1��a) D.
(1��a) D.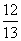 (1��a)
(1��a)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȥ�������������ʵ��Լ���������ǣ�������
A.HNO3��Һ��H2SO4��������BaCl2��Һ������
B.CO2��SO2��������KMnO4��Һ��Ũ���ᣬϴ��
C.KNO3���壨NaCl��������ˮ���ᾧ
D.C2H5OH��CH3COOH����������CaO������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���ʵ�ּ���Ŀ�ĵ���(����)
A����KOH��Һ����SO3(g)��SO2
B����ʪ��⻯�ص�����ֽ����Br2(g)��NO2
C����CO2����NaAlO2��Һ��CH3COONa��Һ
D����BaCl2��Һ����AgNO3��Һ��K2SO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊʵ������ʵ��Ŀ�ģ������±��ṩ����Ҫ�����������Լ���������(����)
| ѡ�� | ʵ��Ŀ�� | ��Ҫ���� | �Լ� |
| A | ����Br2��CCl4�Ļ���� | ��Һ©�����ձ� | Br2��CCl4�Ļ�������ˮ |
| B | ���������Ǻ����� | �Թܡ��ձ����ƾ��� |
|
| C | ʵ������ȡH2 | �Թܡ������ܵ���Ƥ�� | п����ϡHNO3 |
| D | �ⶨNaOH��Һ��Ũ�� | �ζ��ܡ���ƿ���ձ� | NaOH��Һ�� 0��100 0 mol·L��1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г��ӷ�����ȷ���� (����)
A����ȥ��������������ϩ��ͨ��H2���Ӵ�����Ӧ
B����ȥ��������������������ñ���̼������Һϴ�ӣ���Һ
C����ȥNaBr��Һ�е�NaI������ˮ����NaI������CCl4��ȡ��Һ
D����ȥ�Ҵ��������������������ʯ�ң�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����

��ѯ���ϣ����й����ʵ��������±���
| 25 ��ʱ | pHֵ |
| ����H2S��Һ | 3.9 |
| SnS������ȫ | 1.6 |
| FeS��ʼ���� | 3.0 |
| FeS������ȫ | 5.5 |
(1)�����Ƶõ��̷��������Ƿ���Fe3������ѡ�õ��Լ�Ϊ________��
A��KSCN��Һ B��NaOH��Һ
C��KMnO4��Һ D��������Һ
(2)�������У�ͨ�����������͵�Ŀ����____________������Һ���������ữ��pH��2��Ŀ����_______________________________________________________��
(3)��������˳������Ϊ____________����ȴ�ᾧ��_________________________��
(4)�������õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ���___________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com