



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(C2H4O)n![]() nC2H4O��
nC2H4O��
(1)�Ȱѻ�������õ�(C2H4O)n���ٽ����������Һ©�����ֲ�������������� ��
(2)֤���Ƿ����в�����ȩ��������ʵ������������� ��
(3)��������ȩ��Һ����Ũ�����У��������ɺ�ɫ���ʡ����û�ѧ����ʽ��ʾ��һ���̣� ��
(4)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6 mol��L-1 H2SO4�Ļ��Һ����ƿ�з�����ˮ�����Ȼ��Һ�����ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�С�

������ˮ�Ľ����� ����a��b�����������ܵ�Ŀ���� ��
����ƿ�ڵ��ܿڳ��ֵ����ݴ���������Һ��Ĺ����У����Խ��ԽС��ֱ����ȫ��ʧ��������˵����ȩ�ĺ����������ʣ� �����۲쵽�����е������Ѻ�Сʱ����ֹ��Ӧ�ı�Ҫ������ ��Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ȱѻ�������õ�(C2H4O)n�������������Һ©�����ֲ��������������________��
(2)֤���Ƿ����в�����ȩ��������ʵ�������������________________________��
(3)����������ȩ��Һ����Ũ�����У����ɺ�ɫ���ʡ����û�ѧ����ʽ��ʾ��һ���̣�________________________________________________________________��
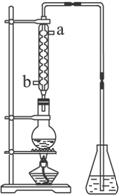
(4)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6 mol��L-1 H2SO4�Ļ��Һ����ƿ������ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�е�ˮ�С�
���������ܵ�Ŀ����________������ˮ�Ľ�����________��(�a����b��)����ƿ�ڵ��ܿڳ��ֵ����ݴ���������Һ��Ĺ����У����Խ��ԽС��ֱ����ȫ��ʧ����һ����˵���Ҵ��ĺ����������ʣ�________�����۲쵽�����е������Ѻ�Сʱ����Ҫ�IJ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��10��)
������ȩͨ��Ϊ40�����ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ�����(C2H4O)n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ(�Եõ���Һ)�����������·�Ӧԭ����
(1)(1.5��)�Ȱѻ�������õ�(C2H4O)n�������������Һ©�����ֲ��������������_____________��
(2) (1.5��)֤���Ƿ����в�����ȩ��������ʵ�������������_______��
(3) (2��)����������ȩ��Һ����Ũ�����У����ɺ�ɫ���ʡ����û�ѧ����ʽ��ʾ��һ���̣� . ��
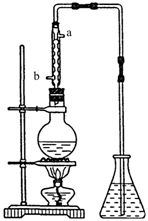 (4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�
(4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�
�� �������ܵ�Ŀ���� ������ˮ�Ľ����� ��(�a����b��)��
�� ��ƿ�ڵ��ܿڳ��ֵ����ݴ���������Һ��Ĺ����У����Խ��ԽС��ֱ����ȫ��ʧ��������˵���Ҵ��ĺ����������ʣ�
���۲쵽�����е�������Сʱ����Ҫ�IJ����ǣ�
�� ��n��3����(C2H4O)n�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com